





Published on Apr 02, 2024
This topic was chosen because of the fascination and the science behind the working of a pigment, and how the colour of each pigment is different and has different shades of the same colour. We wanted to know how to prepare paint pigments using various chemicals and also record the efficiency in producing them.
A pigment is a material that changes the colour of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light. Many materials selectively absorb certain wavelengths of light. Materials that humans have chosen and developed for use as pigments usually have special properties that make them ideal for colouring other materials. A pigment must have a high tinting strength relative to the materials it colours. It must be stable in solid form at ambient temperatures.
For industrial applications, as well as in the arts, permanence and stability are desirable properties. Pigments that are not permanent are called fugitive. Fugitive pigments fade over time, or with exposure to light, while some eventually blacken.
Pigments are used for colouring paint, ink, plastic, fabric, cosmetics, food and other materials. Most pigments used in manufacturing and the visual arts are dry colorants, usually ground into a fine powder. This powder is added to a binder (or vehicle), a relatively neutral or colourless material that suspends the pigment and gives the paint its adhesion. A distinction is usually made between a pigment, which is insoluble in its vehicle (resulting in a suspension), and a dye, which either is itself a liquid or is soluble in its vehicle (resulting in a solution).
A colorant can act as either a pigment or a dye depending on the vehicle involved. In some cases, a pigment can be manufactured from a dye by precipitating a soluble dye with a metallic salt. The resulting pigment is called a lake pigment. The term biological pigment is used for all coloured substances independent of their solubility. Pigments appear the colours they are because they selectively reflect and absorb certain wavelengths of visible light. White light is a roughly equal mixture of the entire spectrum of visible light with a wavelength in a range from about 375 or 400 nanometres to about 760 or 780 nm.
When this light encounters a pigment, parts of the spectrum are absorbed by the molecules or ions of the pigment. In organic pigments such as diazo or phthalocyanine compounds the light is absorbed by the conjugated systems of double bonds in the molecule. Some of the inorganic pigments such as vermilion (mercury sulphide) or Cadmium yellow (cadmium Sulphide) absorb light by transferring an electron from the negative ion (S2- ) to the positive ion (Hg2+ or Cd2+). Such compounds are designated as charge-transfer complexes, with broad absorption bands that subtract most of the colours of the incident white light. The other wavelengths or parts of the spectrum are reflected or scattered. The new reflected light spectrum creates the appearance of a colour. Pigments can only subtract wavelengths from the source light, never add new ones.
The appearance of pigments is intimately connected to the colour of the source light. Sunlight has a high colour temperature, and a fairly uniform spectrum, and is considered a standard for white light. Artificial light sources tend to have great peaks in some parts of their spectrum, and deep valleys in others. Viewed under these conditions, pigments will appear different colours. Colour spaces used to represent colours numerically must specify their light source. Lab colour measurements, unless otherwise noted, assume that the measurement was taken under a D65 light source, or "Daylight 6500 K", which is roughly the colour temperature of sunlight.
The principle behind the working of pigments is related with how different substances have the ability to selectively absorb and reflect different light rays corresponding to their different wavelengths in the visible spectrum. Pigments appear the colours they are because they selectively reflect and absorb certain wavelengths of visible light. White Light is a mixture of all light rays of wavelength 400 nm – 700 nm. When this light encounters a pigment, parts of the spectrum are absorbed by the different components of the pigment. Some other wavelengths or parts of the spectrum are reflected and scattered.
The new reflected light spectrum creates the appearance of a colour. When this reflected light comes in contact with a Human Eye, the Brain perceives the light as the colour corresponding to its Wavelength. Pigments, unlike fluorescent substances can only subtract wavelengths from the source light, and can never add new ones.
To prepare pigments and poster paints using various chemicals and reagents.
1. Clear glue
2.Water
3.Potassium Chromate
4.Lead Nitrate
5.Beakers
6.Iron (III) Chloride
7.Potassium Ferrocyanide
8.Filter Paper
Chemically known as LEAD CHROMATE (PbCrO4) the yellow pigment is obtained as follows:

1. Dissolve 7gm of Potassium Chromate in 50mL water and 10gm Lead Nitrate in 100mL water in two separate beakers.
2. Pour the Potassium Chromate solution in the Lead Nitrate and stir continuously.
3. Lead Chromate separates as precipitate and is the required pigment.
4. Filter the precipitate and dry the pigment.
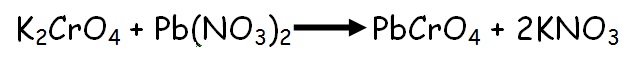
Total mass of reactants used = (Excluding the mass of water used for dilution) =
Mass Of Paint/Pigment Obtained =
Efficiency in formation of yellow colour =
Chemically known as Complex Ferro Cyanide (Fe4[Fe(CN)6]3).

1.Make A solution of 5gm of Hydrated Iron(III) Chloride in 50mL of water.
2. Make a solution of 10gm of Potassium Ferro Cyanide in 75mL of water.
3. Add Iron Chloride solution into Potassium Ferro Cyanide(K4[Fe(CN)6]) solution while stirring briskly.
4. A dark blue colour very fine powdered type substance precipitates.
5.Filter the precipitate and wash it with water. It takes a long time to filter this substance.
![]()
Efficiency Total mass of reactants used = (excluding mass of water used for dilution) =
Mass of Paint/Pigment obtained =
Efficiency In Formation Of Blue Pigment Obtained is =
1.There is tremendous scope for paint pigments in the future.
2.Increasing experimental methods and newer technologies in preparation of pigments has increased the quality and quantity of pigments obtained.
3. Scientists are trying to increase the efficiency of the various paint pigments by newer experimental methods.
4. New shades of colours have been developed in laboratories.
5.Industries have increased their production of pigments and dyes through scientific technology.
6.Pigments are now being used more widely.
7. Natural pigments / non – toxic pigments are being synthesized to avoid any health hazards due to the dangerous chemicals contained in them.
Fine poster paints were obtained using various chemicals and reactants.
1. www.wikipedia.com/Pigments
2. www.google.com
3. www.1000sciencefairprojects.com
4. www.odinity.com/synthesis-malachiteverdigris/
5. www.ionicviper.org
6. www.webexhibits.org
7.www.compoundchem.com