





Published on Apr 02, 2024
Biodiesel refers to a nonpetroleum- based diesel fuel consisting of short chain alkyl (methyl or ethyl) esters, made by transesterification of vegetable oil or animal fat (tallow), which can be used (alone, or blended with conventional petrol diesel) in unmodified diesel-engine vehicles. Biodiesel is distinguished from the straight-vegetable oil (SVO) (sometimes referred to as “waste vegetable oil” “WVO” “used vegetable oil” “UVO” “pure plant oil”, “PPO”) used(alone, or blended) as fuels in some converted diesel vehicles. Biodiesel is standardized as mono-alkyl ester and other kinds of diesel-grade fuels of biological origin are not included.
Fuel-cells have power-generation applications that could utilize biodiesel. Biodiesel can be used in backup systems where the substantial reduction in emissions really matters: hospitals, schools, and other facilities usually located in residential areas. It can also be used to supplement solar power in off-the grid homes.
Fuel cell vehicles turn hydrogen fuel and oxygen into electricity. The electricity then powers an electric motor, just like electricity from batteries powers the motor of an electric vehicle. Fuel cells combine oxygen from the air with hydrogen from the vehicle's fuel tank to produce electricity. When oxygen and hydrogen are combined they give off energy and water (H2O). In fuel cells this is done without any burning (combustion).
Biodiesel has been tested as potential cleaning agent for shorelines contaminated with crude oil, and has been found to increase the recovery of crude oil from artificial sand columns (i.e. the beach). It’s also been used in commercial biosolvents shown to be effective in coagulating crude oil and allowing it to be skimmed off the surface of water.
diesel fuels are required to reduce their sulfur concentration from 500 ppm to 15 ppm. Since sulfur provided most of the fuel’s lubricity, a substitute is required to keep diesel engines functioning properly and avoid premature injection pump wear (i.e. failure). Biodiesel naturally has less than 15 ppm sulfur concentration anyway, and adding just 1 to 2% biodiesel can restore the lubricity to diesel fuel. Apart from these uses, biodiesel can be used in heating our homes, cleaning up tools & grease, removing paint and adhesives, can extend the life of catalytic converters, can be used as the best alternative for the other fuels as it doesn’t produces toxic &harmful substances as compared to other fuels.
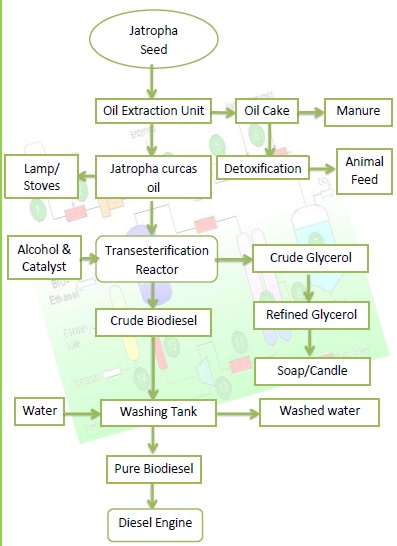
Vegetable Oil
Antifreeze (Methanol)
Lye (NaOH)
Blender
Scales
Plastic Containers
Funnels
Plastic Bottle with lid
Duct Tape
Thermometer
• Measure out 200 ml of antifreeze and put it in one plastic container.
• Add in lye so that the antifreeze is absorbed.
• Cover container and mix well by shaking it. It is mixed when it starts to feel warm and is foamy.
• The mixture has now become sodium methoxide.
• Blend 1 liter of vegetable oil with the sodium methoxide in a blender for 20 minutes.
• Pour mixture into a bottle and wait 8 hours until the byproduct, glycerin, separates form the biodiesel. The glycerin will be on the solid on the bottom.
• Separate out the biodiesel by pouring into a glass bottle.
• Prepare a wash bottle by poking a small hole in the corner of the bottle and covering it with duct tape.
• Wash the biodiesel by pouring it into the wash bottle and adding in ½ a liter of water. Roll the bottle around to mix it and then remove the duct tape and drain the water.
• Repeat the washing process until the biodiesel is clear.This may need to be done numerous times over thecourse of a week to complete the process. Store the biodiesel in a glass container until ready to use.
Biodiesel is currently about one and a half times more expensive than petroleum diesel fuel. Part of this cost is because the most common source of oil is the soybean, which only is only 20% oil. However, the costs of biodiesel can be reduced by making biodiesel from recycled cooking oils rather than from new soy beans, or by making it from plant matter with higher oil content.
It takes energy to produce biodiesel fuel from soy crops, including the energy of sowing, fertilizing and harvesting.
Biodiesel fuel can damage rubber hoses in some engines, particularly in cars built before 1994. You should check with the manufacturer before using biodiesel to see if you need to replace any hoses or rubber seals.
Biodiesel cleans the dirt from the engine. This dirt then collects in the fuel filter, which can clog it. Clogging occurs most often when biodiesel is first used after a period of operation with petroleum diesel, so filters should be changed after the first several hours of biodiesel use.
• htpp://www.icbse.com/
• http://www.sciencedaily.com/
• http://www.biodiesel.org/
• http://www.gas2.org/
• http://www.biofuelsdigest.com/
• http://www.digg.com/