





Published on Apr 02, 2024
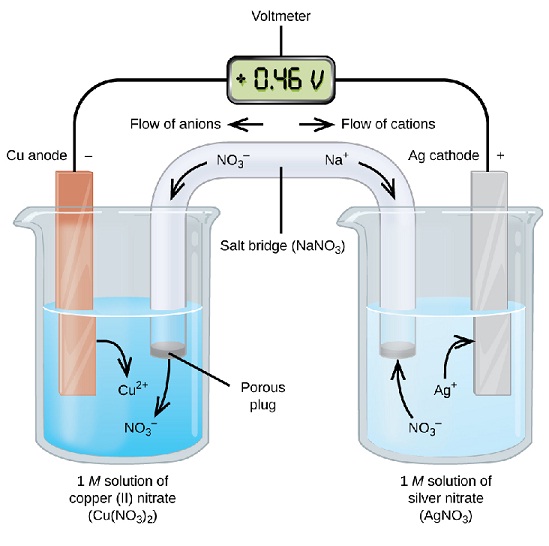
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery". (Actually a single "Galvanic cell"; a battery properly consists of multiple cells, connected in either parallel or series pattern.)
The lemon battery is similar to the first electrical battery invented in 1800 by Alessandro Volta, who used brine (salt water) instead of lemon juice. The lemon battery is described in some textbooks in order to illustrate the type of chemical reaction (oxidation-reduction) that occurs in batteries. The zinc and copper are called the electrodes, and the juice inside the lemon is called the electrolyte. There are many variations of the lemon cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as electrodes. Batteries are used to illustrate the connection between chemistry and electricity as well as to deepen the circuit concept for electricity. The fact that different chemical elements such as copper and zinc are used can be placed in the larger context that the elements do not disappear or break down when they undergo chemical reactions. Batteries serve to illustrate the principles of oxidation-reduction reactions.
This model of the chemical reactions makes several predictions that were examined in experiments published by Jerry Goodisman in 2001. Goodisman notes that numerous recent authors propose chemical reactions for the lemon battery that involve dissolution of the copper electrode into the electrolyte. Goodisman excludes this reaction as being inconsistent with the experiments, and notes that the correct chemistry, which involves the evolution of hydrogen at the copper electrode, has been known for many years. When the electrolyte was modified by adding zinc sulfate (ZnSO4), the voltage from the cell was reduced as predicted using the Nernst equation for the model. The Nernst equation essentially says how much the voltage drops as more zinc sulfate is added.
The addition of copper sulfate (CuSO4) did not affect the voltage. This result is consistent .When the battery is hooked up to an external circuit and a significant electrical current is flowing, the zinc electrode loses mass, as predicted by the zinc oxidation reaction above. Similarly, hydrogen gas evolves as bubbles from the copper electrode. Finally, the voltage from the cell depended upon the acidity of the electrolyte, as measured by its pH; decreasing acidity (and increasing pH) causes the voltage to fall. This effect is also predicted by the Nernst equation; the particular acid that was used (citric, hydrochloric, sulfuric, etc.) doesn't affect the voltage except through the pH value.
Production of current through SELFMADE battery
The Nernst equation prediction failed for strongly acid electrolytes (pH < 3.4), when the zinc electrode dissolves into the electrolyte even when the battery is not providing any current to a circuit. The two oxidation-reduction reactions listed above only occur when electrical charge can be transported through the external circuit. The additional, open-circuit reaction can be observed by the formation of bubbles at the zinc electrode under open-circuit. This effect ultimately limited the voltage of the cells to 1.0 V near room temperature at the highest levels of acidity.
The energy comes from the chemical change in the zinc (or other metal) when it dissolves into the acid. The energy does not come from the lemon or potato. The zinc is oxidized inside the lemon, exchanging some of its electrons with the acid in order to reach a lower energy state, and the energy released provides the power. In current practice, zinc is produced by electron winning of ZnSO4 or pyrometallurgic reduction of zinc with carbon, which requires an energy input. The energy produced in the lemon battery comes from reversing this reaction, recovering some of the energy input during the zinc production.
• Distilled Water, Coldrink, Salt Water
• Connecting Wire
• Copper And Zinc Strips
• Digital Clock
• Assemble a “connection pair” by connecting the wire carefully thread the wire’s exposed metallic end through the holes on the plate. Gently twist wire to secure it to the plate.
• Afterwards, connect the black wire from the LCD clock (negative) to one of the zinc plate. Then connect red wire from LCD clock (positive) to piece of copper plate. Now all the components are connected
• Insert the copper and zinc plates into salt water such that the metallic strips do not touch each other. The clock now starts to work.
• Repeat this experiment with distilled water & coldrink.
As soon as we connect the wires and put the key on electricity generated by the fruit juice flows through the clock, making the clock run in case of salt water and coldrink. The clock does not work when the rods are immersed in distilled water as no current flows.
The metal strips and liquid make a simple battery that creates the electricity to operate the clock. Salty water and coldrink work as a device called electrochemical cell. It converts the chemical energy stored in the metal strips into strips into electrical energy.
A cell works because of the chemical properties of the metals inside (in this case the copper and zinc). The different properties cause tiny particles charged with electricity (ions) to move between the two strips of metal. This flow is an electric current. The liquid which conduct electricity contains the particles that allow the current to flow, but it stops the metals touching. Electric current also flows along the wire between the zinc and copper strips & the clock. This current makes the clock run.
SALT WATER: The ions present in common salt sodium chloride dissociate into ions of sodium and chloride. These ions are responsible for conduction of electricity. Potential is provided by copper and zinc rods.
DISTILLED WATER: There is absence of ions in distilled water therefore the distilled water doesn't conduct electricity and hence the clock doesn’t work. Though the H+ and OH- but the pH is 7 therefore the ion dissociation is not enough only 10-7M H+ is present in distilled water. so this can not conduct electricity.
COLDRINK: The coldrink too contains ions which dissociate to conduct electricity
• NCERT
• Principles of physical chemistry (Puri Sharma)
• hometrainingtools.com
• Wikipedia, the free encyclopedia