





Published on Nov 30, 2023
The invention of transistor enabled the first use of radiometry capsules, which used simple circuits for the internal study of the gastro-intestinal (GI) [1] tract. They couldn't be used as they could transmit only from a single channel and also due to the size of the components. They also suffered from poor reliability, low sensitivity and short lifetimes of the devices.
This led to the application of single-channel telemetry capsules for the detection of disease and abnormalities in the GI tract where restricted area prevented the use of traditional endoscopy.
They were later modified as they had the disadvantage of using laboratory type sensors such as the glass pH electrodes, resistance thermometers, etc. They were also of very large size. The later modification is similar to the above instrument but is smaller in size due to the application of existing semiconductor fabrication technologies. These technologies led to the formation of "MICROELECTRONIC PILL".
Microelectronic pill is basically a multichannel sensor used for remote biomedical measurements using micro technology. This is used for the real-time measurement parameters such as temperature, pH, conductivity and dissolved oxygen. The sensors are fabricated using electron beam and photolithographic pattern integration and were controlled by an application specific integrated circuit (ASIC).
Microelctronic pill consists of 4 sensors (2) which are mounted on two silicon chips (Chip 1 & 2), a control chip (5), a radio transmitter (STD- type 1-7, type2-crystal type-10) & silver oxide batteries (8).
1-access channel, 3-capsule, 4- rubber ring, 6-PCB chip carrier
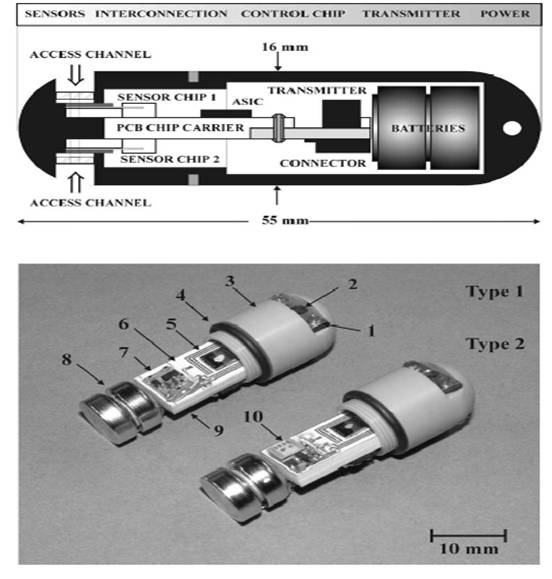
There are basically 4 sensors mounted on two chips- Chip 1 & chip 2. On chip 1(shown in fig 2 a), c), e)), temperature sensor silicon diode (4), pH ISFET sensor (1) and dual electrode conductivity sensor (3) are fabricated. Chip 2 comprises of three electrode electrochemical cell oxygen sensor (2) and optional NiCr resistance thermometer.
An array consisting of both temperature sensor & pH sensor platforms were cut from the wafer & attached onto 100-µm- thick glass cover slip cured on a hot plate. The plate acts as a temporary carrier to assist handling of the device during level 1 of lithography when the electric connections tracks, electrodes bonding pads are defined. Bonding pads provide electrical contact to the external electronic circuit.
• Monotonic temperature response from 1.4 K to 500 K*
• Conformance to standard Curve 10 temperature response curve
• Useful above 60 K in magnetic fields up to 5 T
• The rugged, reliable Lake Shore SD package designed to withstand repeated thermal cycling and minimize sensor self-heating Variety of packaging options
Ion Selective Field Effect Transistor; this type of electrode contains a transistor coated with a chemically sensitive material to measure pH in solution and moist surfaces. As the potential at the chemically active surface changes with the pH, the current induced through the transistor varies. A temperature diode simultaneously monitors the temperature at the sensing surface. The pH meter to a temperature compensated pH reading correlates the change in current and temperature.
This device [5] has an affinity for hydrogen ions, which is the basis for the determination of the pH. The surface of the sensitive area of the sensor contains hydroxyl groups that are bound to an oxide layer. At low pH values hydrogen ions in the sample will bind to these hydroxyl groups resulting in a positively charged surface. In alkaline environments hydrogen ions are abstracted from the hydroxyl groups, leading to a negatively charged surface.
Thus, each pH change has a certain influence on the surface charge. On its turn, this attracts or repulses the electrons flowing between two electrodes in the semiconductor device. The electronics compensates the voltage in order to keep the current between the two electrodes at its set point. In this way this potential change is related to the pH.
Attachment of a polymer membrane on the ISFET introduces the possibility to go beyond the measurement of pH toward other ions. In this plastic layer certain chemicals (ionophores), which can recognize and bind the desired ion, are put in. Now, complex formations of the ionophore and the ion introduce a charge. The potential change is a measure for the ion concentration. Typically, these sensors can be used in a concentration range between app. 10-5 up to 1 mol/l.
Level 1 pattern was defined in 0.9 µm UV3 resist by electron beam lithography. It contains three-electrode electrochemical oxygen sensor & NiCr resistance thermometer.
Most portable or survey instruments used for workplace evaluation of oxygen concentrations make use of "fuel cell" type oxygen sensors. "Fuel cell" oxygen sensors consist of a diffusion barrier, a sensing electrode (cathode) made of a noble metal such as gold or platinum, and a working electrode made of a base metal such as lead or zinc immersed in a basic electrolyte (such as a solution of potassium hydroxide).
To Download Full Report Click Here
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |