





Published on Nov 30, 2023
Energy need has been increasing worldwide exponentially. At present global energy requirements are mostly dependent on the fossil fuels, which eventually lead to foreseeable depletion of limited fossil energy sources. In this context, regeneration of the brewery spent wash is one of the better possibilities. Now a days, countries like India in progressing towards harnessing new energy sources. Fuel cells (FCs) have been thoroughly investigated by many researchers in the past and concluded that FCs could be applied for the treatment of liquid waste streams and also in generation of electricity. From a variety of materials, including complex organic waste and renewable biomass. These sources provide FCs with a great advantage over chemical fuel cells that can utilize only purified reactive fuels (e.g., hydrogen). A developing primary application of FCs is its use in wastewater treatment coupled with electricity generation, although further technical developments are necessary for its practical use. The aim of this study was to test a novel DC-MFC design for distillery wastewater treatment and simultaneous bio-electricity production.
The hypothesis of the design was to bring MFCs closer to large scale industrialization. As such the materials and mechanics of the design were conceived with the consideration of the needs of large scale wastewater treatment plants. Higher percentage of COD and BOD removal and significant electricity generation using MFC can be achieved. Thus the combination of wastewater treatment along with the bio-electricity production may help in saving of millions of rupees. For all the trials conducted in the laboratory, considerable reduction in Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD) and Total Dissolved Solids (TDS) has been achieved. The removal efficiencies ranged between 95.34% to 96.34% for BOD, 90.84% to 98.12% for COD and 40.69% to 59.95% for TDS. The maximum bio- electricity generated was 199.90 mV. Hence the overall reactor efficiency is very encouraging and could be scaled up easily in the near future.
Keywords : DC-MFC, Distillery wastewater, Copper Electrodes.
To characterize distillery wastewater for routine parameters.
To develop and fabricate a novel Dual Chambered Microbial Fuel Cell (DC-MFC).
To evaluate the potential of DC-MFC to treat the distillery wastewater.
To evaluate the potential of DC-MFC to generate bio-electricity with distillery wastewater.
1. Perplex glass material for reactor fabrication
2. Filter media- Milk Marbles and glass wool
3. 4 Copper Electrodes
4. 16-Strands Copper Wire
5. Seeding bottle of 3 litre capacity
6. Aerator
7. Digital Multimeter
The DC-MFC is fabricated with perplex glass of 5mm thickness with the capacity of 5.24 liters. Copper electrodes were used and the positioning of the electrodes were worked out. The reactor was set up by placing the semi-permeable membrane consisting of glass wool and milk marbles between the two aerobic chambers. The inlet chamber was connected with an aerator (Talyomax, T1-100). A three liters capacity bottle was connected to the reactor to seed the distillery wastewater to the reactor with a controlled flow rate and a digital multimeter was connected to record the bio-electricity generated in mV.
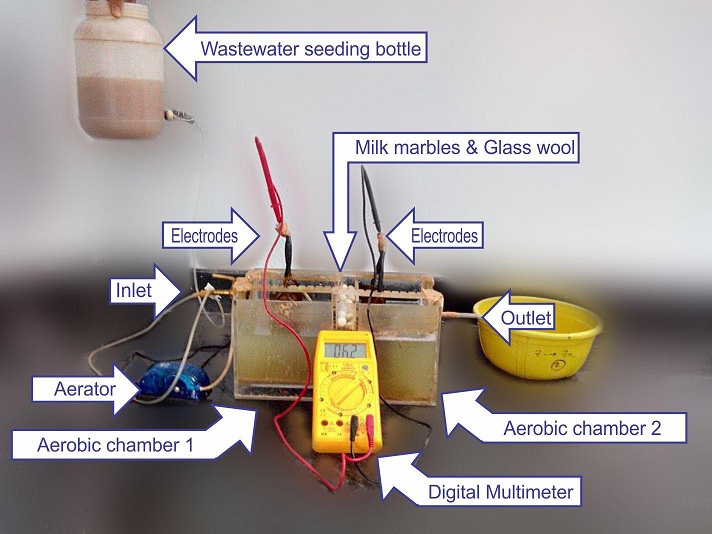
Various parameters considered are:
1. Chemical Oxygen Demand (COD)
2. Biological Oxygen Demand (BOD)
3. Total Dissolved Solids (TDS)
COD is the measurement of oxygen in water consumed for chemical oxidation of pollutants. It determines the quantity of oxygen required to oxidize the organic matter in water or wastewater sample, under specific conditions of oxidizing agent, temperature and time.
BOD determination is the chemical procedure for determining the amount of dissolved oxygen needed by aerobic organisms in a water body to break the organic materials present in the given water sample at certain temperature over a specific period of time.
TDS refers to materials that are completely dissolved in water these solids are filterable in nature. It is defined as residue upon evaporation of filterable sample.
COD digester
Burette and burette stand
COD Vials with Stand
250ml conical flask
Pipettes
Pipette bulb
Tissue Paper
Wash Bottles

Organic Freed Distilled Water
Ferroin Indicator
Potassium Dichromate
Silver Sulphate
Mercury Sulphate
Sulphuric Acid
Ferrous Aluminium Sulphate
Three COD vails with stopper were taken (two for the sample and one for the blank.
Then 2.5 ml of sample was added to each of the two vails and the remaing COD vails for blank; and distilled water was added.
1.5 ml of potassium dichromate reagent - digestion solution was added to each of the three COD vails and 3.5 ml of sulphuric acid reagent – catalyst solution was added in the same manner.
Tubes were closed tightly and the COD digester was switched on and the temperature was maintained at 1500C and time was set for two hours.
Then the COD vails were placed into a block digester at 1500C and heated for two hours.
The vails were removed and allowed to cool at room temperature. The burette was filled with ferrous ammonia sulphate and adjusted to zero and the burette was fixed to the stand.
Contents of the blank vails were transferred to the conical flask.
Few drops of Ferroin indicator were added to the solution and the solution turns into bluish green in colour.
The solution was titrated with the ferrous ammonium sulphate taken in the burette.
End point of titration was indicated by reddish brown colour.
Known volume ferrous ammonium sulphate solution is added for the blank (A) was noted down.
The contents of the sample vail were then transferred into conical flask.
Few drops of Ferroin indicator was added and the solution turns green in colour.
Ferrous ammonium sulphate taken in the burette was then titrated.
End point of the titration was found by appearance of the reddish brown colour.
Volume of the ferrous ammonia sulphate solution added for the sample (B) was noted down.
CHEMICAL OXYGEN DEMAND = ((A – B)* 8 * 1000)/ volume of the sample.
BOD incubator
Burette and burette stand
300 ml glass stopper BOD bottles
500 ml conical flask
Pipettes with elongated tips
Pipette bulb
250 ml graduated cylinders
Wash bottle

Calcium Chloride
Magnesium Chloride
Ferric Chloride
Di potassium Hydrogen Phosphate
Potassium Di Hydrogen Phosphate
Ammonium Chloride
Manganese Sulphate
Potassium hydroxide
Potassium iodide
Sodium indicator
Sodium thiosulphate
Four 300 ml glass stoppered BOD bottles were taken.
10 ml of the sample were added to each of the two BOD bottles and remaining quantities was filled with dilution water.
The remaining two BOD bottle were for blank, dilution water was added to these two bottles.
After the addition glass stopper was placed immediately over the BOD bottles and the number of bottles for identification was noted down.
One blank solution bottle and one sample solution bottle in a BOD incubator at 200 C for 5 days were preserved.
Then the other two bottles (blank and sample) were analyzed immediately.
2 ml of manganese sulphate was added to the BOD bottle by inserting the calibrated pipette just below the surface of the liquid.
Then 2 ml of alkali-iodide-azide reagent is added in the same manner.
The pipette was dipped inside the sample or else oxygen will be introduced to the surface.
Then it was allowed to settle for sufficient time in order to react completely with oxygen.
As soon as this floc has settled to the bottle, shake the contents thoroughly by turning it upside down.
2 ml of concentrated sulphuric acid was added via pipette held just above the surface of the sample.
Then it was stoppered carefully and inverted several times to dissolve the floc.
After the transfer of contents to Erlenmeyer flask, titration was started.
Burette then rinsed with sodium thiosulphate and then filled it with sodium thiosulphate and then the burette was fixed to the stand.
203 ml of solution from the bottle was measured and transferred to an Erlenmeyer flask.
The solution along with standard sodium thiosulphate solution was titrated until the yellow color of liberated iodine was almost faded out and 1ml of starch solution was added.
The titration was then continued until the blue color disappears to colourless.
The volume of sodium thiosulphate solution added which gives the D.O in mg/l was noted down.
This titration was repeated for concordant values.
After five days, the bottle from the BOD incubator and then the sample and the blank for DO was analyzed.
2 ml of manganese sulphate was added to the BOD bottle by inserting the calibrated pipette just below the surface of the liquid.
Then again 2 ml of alkali-iodide-azide reagent was added in the same manner.
If oxygen is present a brownish-orange cloud of precipitate or floc will appear.
It was allowed to settle for sufficient time in order to react completely with oxygen.
When this floc has settled to the bottom the content was shaken thoroughly by turning it upside down.
The reactor capacity could be scaled up to achieve higher bio-electricity generation.
The overall efficiency of the reactor could be tested for different electrode materials.
The efficiency of the reactor to generate bio-electricity could be tested for different types of wastewater.
Initial Characterization of distillery wastewater
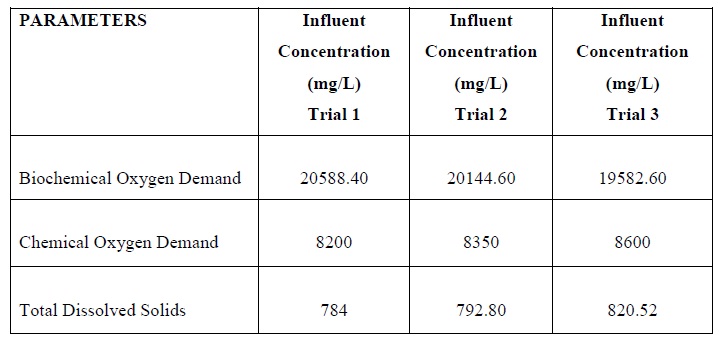
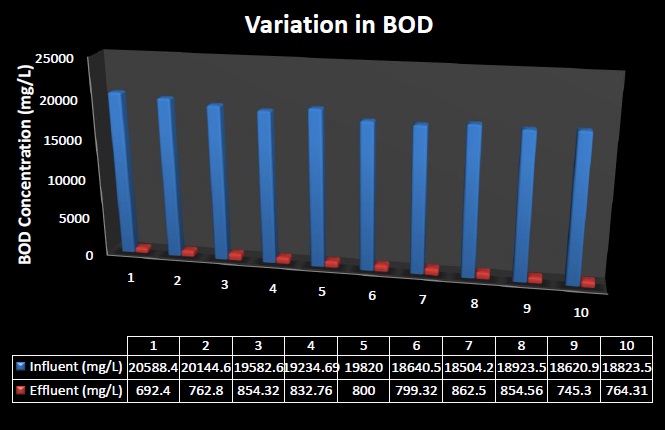
The maximum concentrations of parameters BOD, COD and TDS in the sampled distillery wastewater were found to be 20588.4 mg/L, 9985 mg/L and 892 mg/L respectively.
The design of the rector has been done as per the design details using perplex glass and copper electrodes. It has been proved highly efficient.
After the treatment, concentrations of parameters BOD, COD and TDS, in the effluent of distillery wastewater were found to be 692.40 mg/L, 398 mg/L and 402 mg/L respectively. The considerable Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD) removal efficiency ranged between 95.34% to 96.34% and 90.84% to 98.12% respectively.
After the treatment considerable reduction in Total Dissolved Solids (TDS) concentrations was observed, and the efficiency ranged between 40.69% to 59.95% respectively.
Significant reduction of colour and odour has been achieved.
The work was focused on bio-electricity generation simultaneously and a maximum output power of 199.90 mV was generated.
All though the energy that could be captured from wastewater is not enough to power a city, it is large enough to someday power a treatment plant. With advance, capturing this power could achieve energy sustainability for the water infrastructure.
Abhilasha Singh Mathuriya, V.N. Sharma (2012): Treatment of Brewery Wastewater and Production of Electricity through Microbial Fuel Cell Technology. Journal on Microbial Biotechnology.Volume-1.
Adesoji T, Jaiyeola Joseph, K. Bwapwa (2015): Treatment technology for brewery wastewater & water-scarce country: A review. Journal on Brewery wastewater treatment technology.
Animesh Deval, Anil Kumar Dikshit (2013): Construction, Working and Standardization of Microbial Fuel Cell, PP 59 – 63.
Anupama, Pradeep NV, Hampannavar US (2011): Microbial fuel cell an alternative for COD removal of distillery wastewater. Journal on Research in Biology.