





Published on Nov 30, 2023
Supply of continuous electricity is still not available in several areas of the country and the world. At such places, this work will be helpful for refrigeration of food, medicines, etc... This paper investigates the result of an experimental study carried out to determine the performance of domestic refrigerator when a liquefied petroleum gas (LPG) which is locally available which comprises of 24.4% propane, 56.4% butane and 17.2% isobutene which is varied from company to company is used as a Refrigerant. The LPG is cheaper and possesses an environmental friendly nature with no Ozone Depletion Potential (ODP) and no Global Warming Potential (GDP). It is used in world for cooking purposes.
The refrigerator used in the present study is designed to work on LPG. The performance parameters investigated is the refrigeration effect in certain time. The refrigerator worked efficiently when LPG was used as a refrigerant instead of R134a.
Also from the experiment which done in atmospheric condition, we can predict the optimum value of cooling effect with the suitable operating condition of regulating valve and capillary tube of the system. The use of LPG for refrigeration purpose can be environment friendly since it has no ozone depletion potential (ODP). Usually LPG is used as a fuel for cooking food in houses, restaurants, hotels, etc.. and the combustion products of LPG are CO2 and H2O. In this project we have designed and analyzed a refrigerator using LPG as refrigerant.
LPG is available in cylinders at high pressure. When this high pressure LPG is passed through the capillary tube of small internal diameter, the pressure of LPG is dropped due to expansion and phase change of LPG occurs in an isoenthalpic process. Due to phase change from liquid to gas latent heat is gained by the liquid refrigerant and the temperature drops. In this way LPG can produce refrigerating effect for a confined space. From experimental investigations, we have found that the COP of a refrigerator which uses LPG is higher than a domestic refrigerator.
Due to the huge demand of electricity over the world, we think of recovering the energy which is already spent but not being utilized further, to overcome this crisis with less investment. The climatic change and global warming demand accessible and affordable cooling systems in the form of refrigerators and air conditioners. Annually Billions of dollars are spent in serving this purpose. Hence forth, we suggest COST FREE Cooling Systems. Although government agencies are not able to continuously supply a major portion of electricity in both the urban as well as in rural areas. Still the people in these regions require refrigeration for a variety of socially relevant purposes such as cold storage or storing medical supplies and domestic kitchens this project has the novelty of using LPG instead of electricity for refrigeration. This solution is convenient for refrigeration in regions having scares in electricity.
The term ‘refrigeration’ in a broad sense is used for the process of removing heat (i.e. Cooling) from a substance. It also includes the process of reducing and maintaining the temperature of a body below the general temperature of its surroundings. In other words, the refrigeration means a continued extraction of heat from a body, whose temperature is already below the temperature of its surroundings. For example, if some space (say in cold storage) is to be kept at -2 ᵒC, we must continuously extract heat which flows into it due to leakage through the walls and also the heat, which is brought into it with the articles stored after the temperature is one reduced to -2 ºC. Thus in a refrigerator, heat is virtually being pumped from a lower temperature to a higher temperature. The refrigeration system is known to the man, since the middle nineteenth century.
The scientist, of the time, developed a few stray machines to achieve some pleasure. But it paved the way by inviting the attention of scientist for proper studied and research. They were able to build a reasonably reliable machine by the end of nineteenth century for the refrigeration jobs. But with the advent of efficient rotary compressors and gas turbines, the science of refrigeration reached its present height. Hebrews, Greeks, and Romans places large amounts of snow into storage pits dug into the ground and insulated with wood and straw. The ancient Egyptians filled earthen jars with boiled water and put them their roofs, thus exposing the jars to the night’s cool air. In India, evaporating cooling was employed.
When a liquid vaporizes rapidly, it expands quickly. The rising modules of vapor abruptly increase their kinetic energy and this increase is drawn from the intermediate surroundings of the vapor. These surroundings are therefore cooled. The intermediate stage in the history of cooling foods was to add chemicals like sodium nitrate or potassium nitrate to water causing the temperature to fall. Cooling wine via above method was recorded in 1550. According to the second law thermodynamics, this process can only be performed with the supply of some external work. It is thus obvious, that supply of power (say electrical motor) is regularly required to drive a refrigerator. The substance which work in a heat pump to extract heat from a cold body and to deliver it to a hot body is called “refrigerant”.
The basis of modern refrigeration is the ability of liquids to absorb enormous quantities of heat as they boil and evaporate. Professor William Cullen of the University of Edinburgh demonstrated this in 1755 by placing some water in thermal contact with ether under a receiver of a vacuum pump. The evaporation rate of ether increased due to the vacuum pump and water could be frozen. This process involves two thermodynamic concepts, the vapour pressure and the latent heat. A liquid is in thermal equilibrium with its own vapor at a pressure called the saturation pressure, which depends on the temperature alone. If the pressure is increased for example in a pressure cooker, the water boils at higher temperature. The second concept is that the evaporation of liquid requires latent heat during evaporation. If latent heat is extracted from the liquid, the liquid gets cooled.
The temperature of ether will remain constant as long as the vacuum pump maintains a pressure equal to saturation pressure at the desired temperature. This requires the removal of all the vapors formed due to vaporization. If a lower temperature is desired, then a lower saturation pressure will have to be maintained by the vacuum pump. The component of the modern day refrigeration system where cooling is produced by this method is called evaporator. If this process of cooling is to be made continuous the vapors have to be recycled by condensation to the liquid state. The condensation process requires heat rejection to the surroundings. It can be condensed at atmospheric temperature by increasing its pressure. The process of condensation was learned in the second half of eighteenth century. U.F. Clouet and G. Monge liquefied SO2 in 1780 while van Marum and Van Troostwijk liquefied NH3 in 1787. Hence, a compressor is required to maintain a high pressure so that the evaporating vapours can condense at a temperature greater than that of the surroundings.
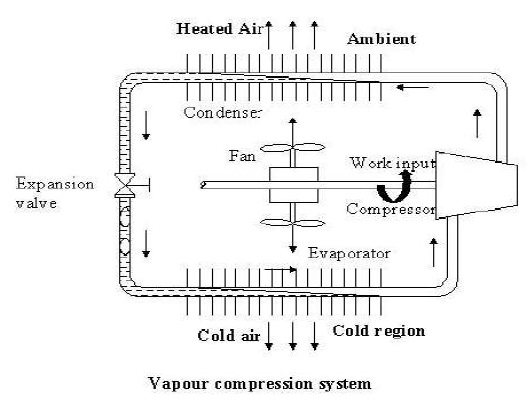
A vapour compression refrigeration system is an improved type of air refrigeration system in which a suitable working substance, termed as refrigerant is used. It condensed and evaporates at temperatures and pressures close to the atmospheric conditions. The refrigerants usually used for this purpose are ammonia, carbon dioxide and sulphur dioxide.
Thermodynamic cycles can be categorized into gas cycles and vapour cycles. In a typical gas cycle, the working fluid (a gas) does not undergo phase change, consequently the operating cycle will be away from the vapour dome. In gas cycles, heat rejection and refrigeration take place as the gas undergoes sensible cooling and heating. In a vapour cycle the working fluid undergoes phase change and refrigeration effect is due to the vaporization of refrigerant liquid. If the refrigerant is a pure substance then its temperature remains constant during the phase change processes.. Hence, the required mass flow rates for a given refrigeration capacity will be much smaller compared to a gas cycle. Vapour cycles can be subdivided into vapour compression systems, vapour absorption systems, vapour jet systems etc. Among these the vapour compression refrigeration systems are predominant.
Compression refrigeration cycles take advantage of the fact that highly compressed fluids at a certain temperature tend to get colder when they are allowed to expand. If the pressure change is high enough, then the compressed gas will be hotter than our source of cooling (outside air, for instance) and the expanded gas will be cooler than our desired cold temperature. In this case, fluid is used to cool a low temperature environment and reject the heat to a high temperature environment. Vapour compression refrigeration cycles have two advantages. First, a large amount of thermal energy is required to change a liquid to a vapor, and therefore a lot of heat can be removed from the airconditioned space. Second, the isothermal nature of the vaporization allows extraction of heat without raising the Simple Vapour Compression Refrigeration System temperature of the working fluid to the temperature of whatever is being cooled. This means that the heat transfer rate remains high, because the closer the working fluid temperature approaches that of the surroundings, the lower the rate of heat transfer.
The refrigeration cycle is shown in Figure below and can be broken down into the following stages:
In the evaporator absorbs heat from its surroundings, usually air, water or some other process liquid. During this process it changes its state from a liquid to a gas, and at the evaporator exit is slightly superheated.
Enters the compressor where its pressure is raised. The temperature will also increase, because a proportion of the energy put into the compression process is transferred to the refrigerant.
Passes from the compressor into the condenser. The initial part of the cooling process (3-3a) superheats the gas before it is then turned back into liquid (3a-3b). The cooling for this process is usually achieved by using air or water. A further reduction in temperature happens in the pipe work and liquid receiver (3b - 4), so that the refrigerant liquid is sub-cooled as it enters the expansion device.
Passes through the expansion device, which both reduces its pressure and controls the flow into the evaporator.
It is shown on T-S below at point 1, let T1, P1, and s1 be the properties of vapour refrigerant . the four processes of the cycle are as follows:
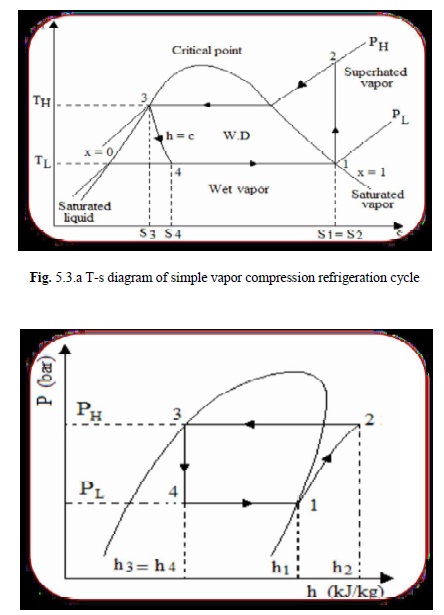
The vapour refrigerant at low pressure p1 and temperature T1 is compressed isentropically to dry saturated vapour as shown by the vertical line 1-2 on T-s diagram and by the curve 1-2 on p-h diagram. The pressure and temperature rises from 1 to 2. The work done during isentropic compression is given by:
W=h2-h1……………………………………….1
The high pressure and temperature vapour refrigerant from the compressor is passed through the condenser where it is completely condensed at constant pressure p2 and temperature T2. The vapour refrigerant is changed into liquid refrigerant. The refrigerant while passing through the condenser, gives its latent heat to the surrounding condensing medium.
The liquid refrigerant at pressure p3=p2 expanded by throttling process through the expansion valve to a low pressure p4=p1 and temperature T4=T1. Some of the liquid refrigerant evaporates as it passes through expansion valve, but the greater portion is vaporized in the evaporator. During the throttling process no heat is absorbed or rejected by the liquid refrigerant.
The liquid vapour mixture of the refrigerant at pressure p4=p1 and temperature T4=T1 is evaporated and changed into vapour refrigerant at constant pressure and temperature. During evaporation, the liquid vapour refrigerant absorbs its latent heat of vaporization from medium (air, water or brine) which is to be cooled. The heat absorbed or extracted by the liquid vapour refrigerant during evaporation is given by:
RE=h1-h4=h1-hf3……………………………….2
Where hf3 is sensible heat at T3 (enthalpy of liquid refrigerant leaving the condenser). The coefficient of performance is ratio of refrigerating effect to the work done.
C.O.P = (h1-h4 ) / ( h1-hf3) = (h1-hf3) / (h2-h1)
Large amount of refrigeration at lower initial purchase and operating cost.
Very efficient
Very compact system for small to very large heat loads.
Cycle can be reversed for heat pump operation.
Parts can wear out.
System causes noises.
Potential refrigerant leaks.
Operates in limited orientation.
Household refrigerator.
Air conditioners.
Water coolers.
Ice and Ice cream makers.
Deep freezers.
Large industrial refrigeration.
Air conditioning systems.
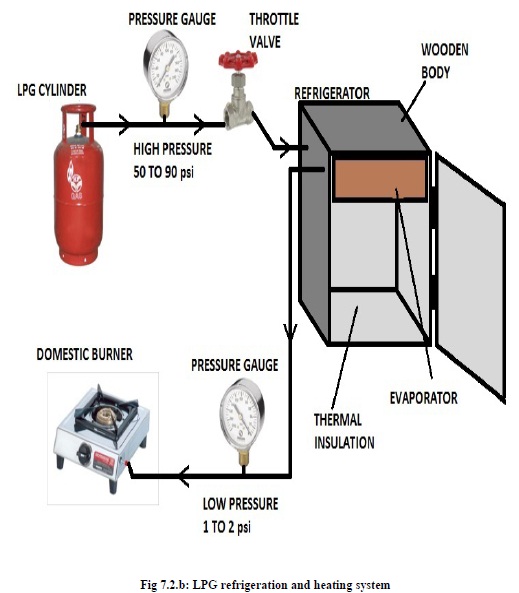
The LPG refrigerator is shown in the figure. We make the one box of the Thermo-coal sheet. The thermo-coal sheet size is 15mm used for the LPG refrigerator. The size of the evaporator is 355*254*152 mm³. We kept the thermo-coal sheet because the cold air cannot transfer from inside to outside of refrigerator. And the evaporator is wrapped totally with aluminum tape. The schematically diagram of the LPG refrigeration system is shown in below diagram. The gas cylinder is connected to high pressure regulator, which is connected to high pressure pipes. To the other end of the high pressure pipes pressure guage is connected. To another end a copper tube is connected which is connected to the capillary tube. The capillary tube is fitted with evaporator. The evaporator coil end is connected to the stove by another high pressure pipe. One pressure guage is put between capillary tube and cylinder and another is put at the end of the evaporator.

The basic idea behind LPG refrigerator is to use the LPG to absorb heat. The simple mechanism of the LPG refrigeration working is shown in the figure below.
LPG is stored in the LPG cylinder under high pressure. When the gas tank of regulators is opened then high pressure LPG passes through the high pressure pipe. This LPG is going by high pressure gas pipe to capillary tube.
High pressure LPG is converted in low pressure at capillary tube with enthalpy remains constant.
After capillary tube, low pressure LPG is passed through the evaporator. LPG is converted into low pressure and temperature vapor from and passes the evaporator which absorbs heat from the chamber. Thus the chamber becomes cool down. Thus we can achieve cooling effect in refrigerator.
After passing through the evaporator low pressure LPG is passed through the pipe to burner. And we can use the low pressure of LPG in burning processes. The LPG Refrigerator is work on the simple Vapour Compression Refrigeration system.
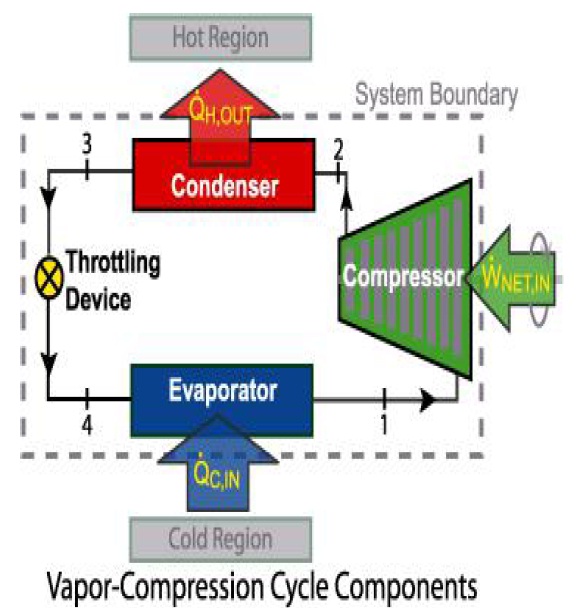
The working of VCR system is as follows:
When the compressor is started, it draws the low pressure vapour from the evaporator at state 2 and compresses it isentropically to sufficiently high pressure up to state 3. Since in compression work is done on the vapour, its temp also increases and hence it is converted into low pressure adiabatically i.e. enthalpy remains constant. After capillary tube, this low pressure LPG is passed through evaporator. In the evaporator LPG is converted into low pressure and temperature form which it absorbs the heat from the cooling chamber. Thus the cooling chamber cools down.
Hot vapour from compressor under pressure is discharged into the condenser where condenser cooling medium usually water or surrounding air is absorb the heat from hot vapour. This converts the hot vapour into liquid and the liquid is collected in liquid receiver at state 4.
The liquid from the liquid receiver at high pressure is then piped to a refrigerant control valve which regulates the flow of liquid into the evaporator. This control valve, while restricting the flow, also reduces the pressure of the liquid with the result the liquid change into vapour of low dryness fraction represented by state 1. During this process the temperature of the refrigerant reduces corresponding to its pressure.
Finally, the low pressure, low temperature refrigerant passes through the evaporator coil where it absorb its latent heat from the cold chamber or from brine solution at constant pressure and converts into vapour at state 2. It is again supplied to compressor. Thus, the cycle is completed.
The idea behind working of LPG refrigeration is to absorb heat from surrounding by using the evaporation of a LPG. The pressure of LPG which is stored in cylinder is at about 80 psi. We are lowering this pressure of LPG up to pressure 15 psi by using capillary and so that cooling is done on surrounding by absorbing heat isentropically. Pressure of LPG in cylinder is high, when the regulator of gas tank is opened then high pressure LPG passes through gas pipe. After that this high pressure LPG goes in the capillary tube from high pressure pipe. In the capillary tube this high pressure LPG is converted into low pressure and hence low temperature because of expansion of LPG gas in capillary tube. Thus we can get refrigerating effect in refrigerator. After that the low pressure LPG from evaporator is passed to the burner through high pressure pipe and we can use this low pressure LPG for burning for further application. In this project we use recompressed LPG cylinder instead of compressor. In this way we can achieve refrigerating effect from this system.
The aim of the LPG refrigerator was to use LPG as a refrigerant and utilizing the energy of the high pressure in the cylinder for producing the refrigerating effect. We have the LPG at a pressure of 12.41 bar in Domestic 14.5 kg cylinder equipped with a high pressure regulator and this pressure has reduced up to 1.41 bar with the help of capillary tube. But if we use a low pressure regulator as is the practice in conventional domestic LPG gas stove, the pressure of LPG after the expansion device and before the burner would be different. So we have calculated the refrigerating effect with the help of changes in properties of LPG (pressure, temperature, and enthalpy) before and after the evaporator using high pressure regulator and the amount of refrigerating effect is determined. With this energy input the COP of the LPG refrigerator is 5.08 and it is greater than the domestic refrigerator. But in the future scope the result may differ if energy input for 1Kg of LPG production, would be taken from the energy audit report of any refinery. This system is cheaper at initial as well as running cost. It does not require an external energy sources to run the system and no moving part in the system. So maintenance cost is also very low. This system is most suitable for hotel, industries, refinery, chemical industries where consumption of LPG is very high.
We conclude that:
Propane is an attractive and environmentally friendly alternative to CFCs used currently.
Mass flow rate increases with increase in capillary inner diameter rand coil diameter where as mass flow rate decreases with increase in length. It was observed that the COP of system increases with similar change in geometry of capillary tube.
Cooling capacities were obtained order of about three- to four fold higher for LPG than those for R- 12. capillary tube. COP of LPG refrigerator was higher than that of R134a by about7.6%. LPG seems to be an appropriate long-term candidate toreplaceR134a in the existing refrigerator,
High COP values were obtained No operation problems have been encountered compressor. The use of LPG as a replacement refrigerant can contribute to the solution of (ODP) problem and global warming potential.
[1] Zainal Zakaria and Zulaikha Shahrun “ The possibility of using liquefied petroleum gas in domestic refrigeration system” International Journal of Research and Reviews in Applied Science(IJRRAS), December 2011, Volume9
[2] Vishwadipsingh J. Ghariya and Swastik R. Gajjar “International Journal for Scientific Research and Development” ISSN (online): 2321-0613, March 2014, Vol.2
[3] Ibrahim Hussain Shah and Kundan Gupta “International Journal of Engineering Sciences and Research Technology” ISSN: 2277-9655, July 2014, Vol. 3(206- 213).
[4] Khandare R. S. and Bhane A. B “International Journal of Emerging Technology and Advanced Engineering” ISSN: 2250-2459, March 2015, Volume 5.