





Published on Nov 30, 2023
The increase of pollutant concentrations in water resources is one of the most serious environmental problems worldwide. Heavy metals are one of the major chemical pollutants that cause acute toxicity and have a long-term accumulation in the environment. The presence of heavy metals, such as lead, in water has been a public concern during the last decade. The wastewater from different industries including electroplating, metallurgical, nuclear, wood and paper, painting, and dyeing contains a large amount of lead ions .Among the different adsorbent materials used for heavy metals removal. Nano-sized materials are being widely used due to their properties. Carbon nanotubes, nano-metal oxides, nano-zeolite composites, polymers, and polymer-metal oxides are some of the adsorbents used for this purpose. Nano-metal oxides are a group of nanomaterials whose application for adsorption purposes is increasing due to their unique properties. Among the nano-metal oxides, TiO2 has unique physical and chemical properties such as non-toxicity, large surface area and photocatalysis.
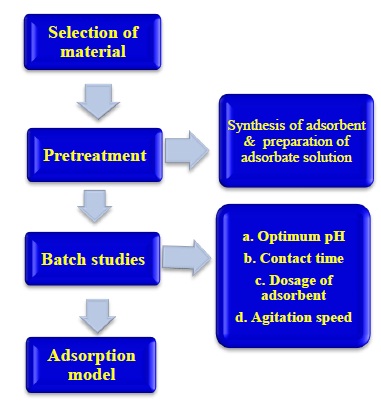
In this study, the sol-gel method has been applied to synthesize TiO2 nanoparticles. In this method, the nanoparticles were uniform both in size and shape and the synthesis procedure was simple to use. The aim of this study was to use synthesized nano TiO2 for the adsorption of lead from aqueous solution under batch conditions. The structure of the synthesized nanoparticles was characterized using XRD. Moreover, the effects of pH, contact time and adsorbent dosage on adsorption process were investigated.
Keywords : Titanium dioxide (tio2), lead removal from aqueous solution, freundlich and langmuir isotherm models.
The main objective is to evaluate the Nano TiO2 in removing lead ions (Pb+2) from aqueous solution.
Specific objectives:
1. To synthesis Nano TiO2 particles by sol-gel method.
2. To study and evaluate the efficiency of Nano TiO2 in the removal of lead (Pb+2) through batch experiments.
3. To analyze adsorption isotherm and identify suitable isotherm.
The stock solutions for preparation of lead solution were prepared by dissolving Pb(NO)3 in deionized water. TiCl4 and NH4 were used to synthesize TiO2 nanoparticles by the sol-gel method. For adjusting pH, 1 M HNO3, NaOH is used. The apparatus used are Hot air oven, Muffle furnace, Magnetic stirrer, Centrifuge and U V Spectrophotometer.
Synthesis of Nano TiO2 - 30 ml of Ticl4 was mixed with 1 litre of distilled water by vigorous stirring at 800 rpm.
The pH of the above solution was adjusted to 7 by adding NH3 dropwise and mixing was continued until gel was formed.
The gel was left for 7 days until colloidal sediment of TiO4 was formed.
Then TiO2 sediment was separated by filtration.
The separated sediment was placed in oven for 24 hours at 70ºC.
Then calcination was performed at 400ºC for 4 hours.
About 1.615 g of Pb(NO)3 was dissolved in 1000 ml distilled water. Solutions of different dilutions required for the adsorption studies were prepared by diution of stock solutions.
A batch experiments were carried out in order to investigate the effect of various parameters.
The various parameters tested are 1. Adsorption dosage 2. Contact time 3.Optimum pH and 4. Shaking speed.
Once the adsorbent was prepared, it was added to 50 ml of stock solution and placed on the magnetic stirrer for a contact time of 30 min and the pH 7
Then to test the removal efficiency of the stock solution, the sample was placed in the centrifuge for 10 min at a 300 rpm so that the adsorbent particles get separated from the treated solution.
UV Spectrophotometer was used for the analysis of final concentration of the effluent.
The results indicated that Tio2 could be an effective adsorbent for the adsorption of Pb2+ ions from the aqueous solution under optimized conditions of pH 5, adsorbent dosage of 2 mg/L and a contact time of 40 min.
The method may be tested for removal of other heavy metal ions from industrial wastewater.
Pilot-plant studies would benefit the field application of this process.
Recovery of valuable metals and reuse of adsorbent may be studied in detail.
Detailed investigations are needed for the safe disposal of adsorbent used.
The morphology of Tio2 and its effects on removal of heavy metals can also be studied.
Column studies can be tested and its adsorption efficiency can be studied.
Adsorbent characterisation of crystalline size of the adsorbent was determined by X- Ray diffraction. For the TiO2, Langmuir isotherm was found to be best suited with R2 = 0.9021. The adsorption isotherms for Pb+2 fitted well to the Langmuir equation than the Freundlich isotherm. Since here the adsorbent was used after chemical treatment, initial uptake of Pb+2 could be mainly due to chemisorption rather than Physisorption which is evident from the Langmuir equation.