





Published on Nov 30, 2023
Nanoparticles of Magnesium Oxide (MgO) (also known as “nano metal oxide”) were synthesized by using Solution Combustion Technique, using Magnesium Nitrate as oxidizer and sugar as fuel. Grab samples collected at the inlet and outlet of M/s Karnataka Soaps and Detergents Limited, Bengaluru were characterised using various parameters. Analysis of all parameters was carried out as per the “Standard Methods for the Examination of Water and Wastewater, APHA, AWWA ad WEF, 2012”. Batch shaking process was performed using MgO as adsorbent in the treatment of wastewater. It was found that 0.25g of MgO powder could remove 91% of COD, 6.2% Chloride and 93.7% of BOD from the inlet sample.
Recent advances in nanotechnology offer leapfrogging opportunities to develop next-generation water treatment systems. Our current water treatment, distribution and discharge practices, which heavily rely on conveyance and centralized systems, are no longer sustainable. The highly efficient, modular and multi-functional processes enabled by nanotechnology as envisaged to provide high performance, affordable water and wastewater treatment solutions that rely less on large infrastructures.
Researchers have long tried to come up with simpler and effective nanoaparticles synthesis routes. T. Mimani and K. C. Patil (2001), investigated the production of various nanoparticles of metal oxides by using a technique known as, “Solution Combustion Synthesis”. Xiaolei Qu, Pedro J.J, Alvarez, Qilin Li (2013), provided an overview of recent advances in nanotechnologies for water and wastewater treatment. The major applications of nanomaterials were critically reviewed based on their function in unit processes. Three categories of the technologies show most promise of full-scale application, namely, nano-adsorbents, nanotechnology enabled membranes and nano-photocatalysts. The barriers for their full-scale application and the research needs for overcoming these barriers were also discussed. B Nagappa and G T Chandrappa (2007) studied the COD removal from Paper Mill Effluent by using nanocrystalline Calcium Oxide (CaO) as adsorbent.
Nanosized metal oxides such as MgO, CaO, ZnO, etc., are excellent adsorbents for a wide variety of adsorbates. The use of nanoscale MgO has been decided as it is non-toxic and environmental friendly. The preparation method adopted is cost-effective and doesn’t consume much time.
Keywords : Solution Combustion Synthesis, Nanoscale MgO, Adsorption, Organic Pollution Removal
In this project, an interdisciplinary approach to wastewater treatment by removing contaminants at the nano scale was undertaken. The project aims at encouraging alternative treatment techniques to sustain the increasing the water demand. The specific objectives include:
1. To improve treatment efficiency and reduce the time taken for satisfactory removal of organic and inorganic contaminants. Therefore, large quantities of wastewater can be treated.
2. To minimize the quantity of chemicals used (Application of metal oxide nanomaterials is in terms of milligrams as compared to the conventional kilogram).
3. To regenerate the metal oxide nanomaterial used and recycle it to a desirable extent ( 3-4 times). After a number of regeneration and reuse cycles, the nanomaterial can be used as a raw material in various industrial processes.
4. To minimize the amount of solid waste generated at the end of the treatment process (up to 90 %).
The high adsorption capacity, low cost, easy separation and regeneration make metal based nano-adsorbents technologically and economically advantageous.
In this project, chemicals and reagents of AR grade and distilled water were used in the preparation of solutions. Porous nanocrystalline MgO was prepared by Solution Combustion Synthesis process. Aqueous solution of Magnesium Nitrate as oxidizer and sugar as fuel (corresponding to equivalent stoichiometric ratio (Øc) of oxidizer to fuel (O/F) = 0.21) was taken in a beaker. The excess water was allowed to evaporate by heating on a hot plate until a gelatinous mass was left out and then introduced into a muffle furnace maintained at 450 ± 10 °C. Initially, the gelatinous mass underwent dehydration. Later, flameless ignition (smoldering) appeared at one end and propagated throughout the mass within a couple of minutes. Voluminous and porous nanocrystalline, pale white MgO was obtained. The theoretical equation assuming complete combustion of redox mixture used for the synthesis of MgO maybe written as:
24Mg(NO3)2 (aq) + 5C12H22O11(aq) ----> 24MgO(s) + 24N2 (g) + 60 CO2 (g) + 55H2O (g)
Grab samples of soaps and detergents manufacturing unit (courtesy of M/s Karnataka Soaps and Detergents Limited, Bengaluru) were collected at the inlet and outlet of their Effluent Treatment Plant (ETP) in clean plastic cans and stored at 4°C according to the “Standard Methods for the Examination of Water and Wastewater, APHA, AWWA ad WEF, 2012”. Characterization of these samples was carried out as per the standard methods prescribed in the abovementioned Standard Operating Procedure. Parameters such as pH, Conductivity, Tota Suspended Solids (TSS), Total Dissolved Solids (TDS), Chloride, Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD) as well as Oil and Grease were analyzed.
The organic pollution removal experiments were performed with inlet sample of the soaps and detergents manufacturing unit; utilizing batch stirring process. In each experiment 100 mL of inlet sample was taken in 250 mL beaker; a known quantity of MgO (0.05 – 0.3 g) as adsorbent was added. In batch experiments, the optimum values o variables such as pH (the process is independent of pH), stirring time (20 minutes) and settling time (15 minutes) were maintained. The organic content adsorbed by MgO settled at the bottom of the beaker was separated by decantation and is termed as sludge. The residual organic and chloride content was measured as COD, BOD and chloride values in accordance to the Open Reflux Method, Titrimetric Method and Ergentometric Method, respectively, as suggested in the standard manual. The amount of sludge deposited was also measured.
General information regarding the soaps and detergents manufacturing unit is as shown in Table 1. Grab samples collected at the inlet and outlet of the effluent treatment plant were characterized using various parameters using standard methods as given below. From the results (Fig. ), it is evident that while the all parameters, apart from Conductivity, TDS and Chloride are maintained below the E (P) Tolerance Limits.
Different quantities (0.05 – 0.3 g) of MgO (as adsorbent) was taken separately in 100 mL of inlet sample in six beakers and kept for batch shaking on a magnetic stirrer. Thereafter, the measurement of organic content of the treated effluent was performed and a relationship between COD removal capacity (percentage) and adsorbent quantity (g) was established as shown in Figure 1. Similarly, removal efficiencies of BOD and Chloride for each dosage of MgO was also calculated, as shown in Figure 2 and Figure 3, respectively. From the trend in Figure 1, it can be seen that the maximum level of COD removal occurs in the presence of 0.3 g of MgO. After this, removal rate becomes almost constant. With the dosage of 0.25 g of MgO, about 1714 mg/L (¬91%) of COD could be removed from total 1883 mg/L and this value is within the limit (250 mg/L) of Environment (Protection) Rules, 1986. Hence, 0.25g of MgO is considered as the optimum dosage for the subsequent experimental studies. However, there is an increasmge in pH and hardness after treatment and this increase is due to the partial dissolution of MgO and formation of MgCO3.
From Figure 2, we can infer that the maximum level of BOD removal occurs in the presence of 0.3 g of MgO. With the dosage of 0.25 g of MgO, about 1350 mg/L (¬93.7%) of BOD could be removed from total 1440 mg/L and this value is within the limit (100 mg/L) of Environment (Protection) Rules, 1986. Hence, 0.25g of MgO is confirmed as the optimum dosage.
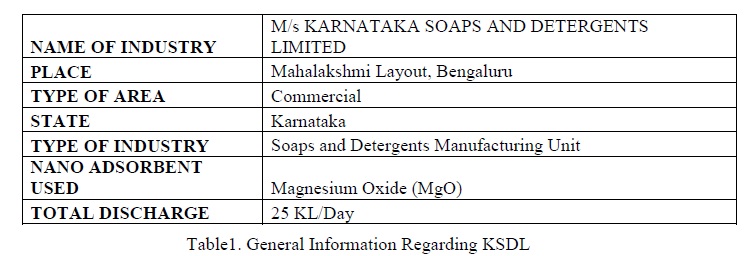
From Figure 3, we can see that the maximum level of chloride removal occurs in the presence of 0.2 g of MgO. At this dosage about 48.6 mg/L (¬6.2%) of chloride could be removed from total 481 mg/L and this value is within the limit (00 mg/L) of Environment (Protection) Rules, 1986.
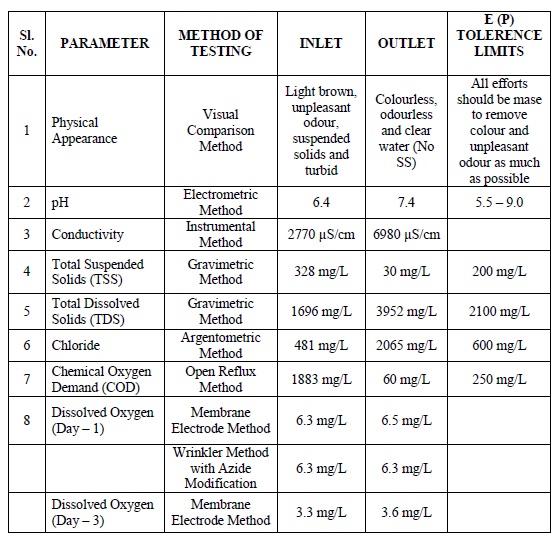
The effect of contact time on COD removal rate of adsorption was determined by using optimum dosage of 0.25g of MgO powder. The batch experiments for the removal of COD were conducted at different intervals of stirring time in 100 mL of inlet sample. With every 10 minutes of stirring, the residual COD in treated water was measured. The COD removal gradually increased with time and attained maximum value after 20 minutes stirring (Fig 4) and this duration could be considered as optimum time for highest level of COD removal. The effect of pH (5-9) on COD removal was carried out using 0.1 M NaOH solution and 0.25g of MgO with 20 minutes stirring time (Fig 5). It is evident that COD removal is independent of pH.
This technology can be further applied for the treatment of various industrial effluent, where conventional/existing treatment technique does not meet the stipulated E (P) Rules, 1986 Tolerance Limits. It can be applied in the existing ETPs to increase the efficiency of the treatment at an appropriate stage without disturbing the existing method in place. Nano-adsorbents can be readily integrated into existing treatment processes in slurry reactors or adsorbers. Applied in powder form, nano-adsorbents in slurry reactors can be highly efficient since all surfaces of the adsorbents are utilized and the mixing greatly facilitates mass transfer. However, an additional separation unit is required to recover the nanoparticles. Nano-adsorbents can also be used in fixed or fluidized adsorbers in the form of pellets/beads or porous granules loaded with nano-adsorbents. Fixed-bed reactors are usually associated with mass transfer limitations and head loss; but it doesn’t need a future separation unit.
In conclusion, a novel combustion synthesis method has been used successfully to prepare nano scale MgO powder. Magnesium nitrate plays dual role as metal source and oxidizer, and sugar acts as a fuel for the combustion process. Since Øc = 0.2, the mixture is said to be fuel rich. It has been demonstrated that nanocrystalline metal oxides have unparalleled sorption properties for organics/inorganics owing to their extremely high specific surface area and associated sorption sites, pore size and surface chemistry. From the synthesis of nano scale MgO to the recovery and reuse, it is a cost-effective and environmental friendly method of wastewater treatment.