





Published on Apr 02, 2024
My aim in this scientific investigation is to see how concentrates of vinegar (H20 + CH302H) mixed with water effect the weight of limestone ( CaC03- Calcium carbonate i.e. chalk, marble) over a period of time. We will look at the change and decrease in the weight, over the time of 5 minutes.
This experiment is based on acid rain, but what is acid rain? It is a broad term referring to more than natural amount of sulfuric acid and nitric oxide. Acid rain is referring to rain with a pH4 or lower. Natural causes are lightning strikes, volcano eruptions and also dead and decaying foods. However, the main effect is man-made, burning of the coal; this lets of sulfuric oxides, the other one is cars, trucks etc…; they let nitric acid into the atmosphere. However, we will be substituting this with vinegar.
The technical way it affects the limestone is the neutralizing reaction (because vinegar is acid and limestone is alkali) is the CaC03( Calcium Carbonate) reacting with H2S04 (Sulfuric acid) = CaSO4 ( Gypsum) + H2CO3 ( Carbonic acid) results in production of CO2 gas. And this whole neutralization reaction results inthe limestone dissolving and crumbling. This reaction can be mimicked by using vinegar on egg shells.Limestone will neutralize strong acids, at least partially. However, in real-life application limestone cannot be depends on to neutralize acidic polluted water because they chips are very easily
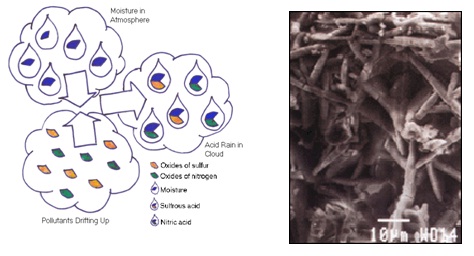
When, sulfuric, and nitric acids react with the calcite limestone, the calcite melts. It exposed areas of buildings and statues, this result in roughened surfaces, less of material, and loss of carved details. This black crust is mainlymade of gypsum, a mineral that forms from the reaction between calcium carbonate and the sulfuric acid. It remains on surfaces that are protected from the rain. Gypsum is white, but the crystals form networks that trap particles of dirt and pollutants, so the crust looks black. Eventually the black crust peels off, leaving crumbling stone.
How different concentrations of vinegar mixed with water effect the weight of the limestone over 5 minutes?
I think that the beaker with most concentrate of vinegar will reduce the weight of the lime stonethe most. Because in my background information, I can understand that the more of the neutralization reaction there is the more crumbling, dissolving and forming of the gypsum. I think that the beaker with a more acidic substance (15ml of vinegar and 5ml of water) will reduce the weight of the limestone by the most.
Independent variable: The Concentrate of Vinegar
Dependent Variable: The weight of the limestone (after the reaction)
Constant Variable: The original weight of the limestone
1. Weighing Machine
2. Flask-50ml x6
3. Limestone- 2g x 6
4. Vinegar- 30ml
5. Water
6. Test Tubes
7. Plastic Box
8. Stopwatch
1) Fill the flask with 2g of limestone.
2) Fill the test tube with the different quantity of liquid being used (Ex. 15ml vinegar, 5ml H2O)
3) Place the flask with 2g of limestone on the weighing machine.
4) Pour the liquid in the test tube into the flask and take the initial weight.
5) Take the change of the weight every 30 seconds to see if it increases, decreases or stays the same for 5 minutes with a stopwatch.
6) Then do this again with different quantities of liquids in the test tubes (ex. 10ml vinegar, 10ml water)
7) Repeat steps 1 to 6 two or three times to get reliable results
• Don’t throw materials around and play around with things.
• Be careful with all tools and always help others in need.
• Don’t eat, drink, or smoke while in the laboratory.
• Don’t perform any unauthorized work.
• Don’t pour any liquids that aren’t water down the sink.
• Don’t point the open end of a test tube at yourself or somebody else.
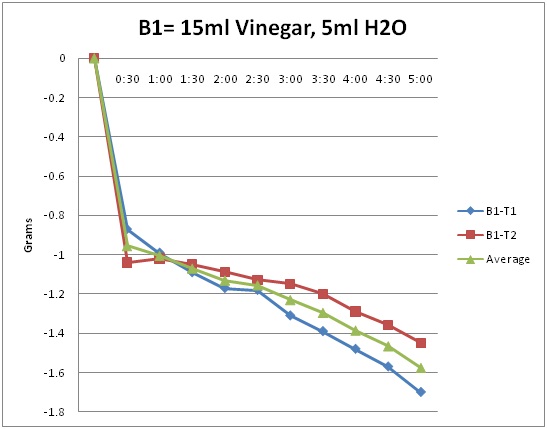
B1= 15ml Vinegar, 5ml H2O
B2= 1Oml Vinegar, 10ml H2O
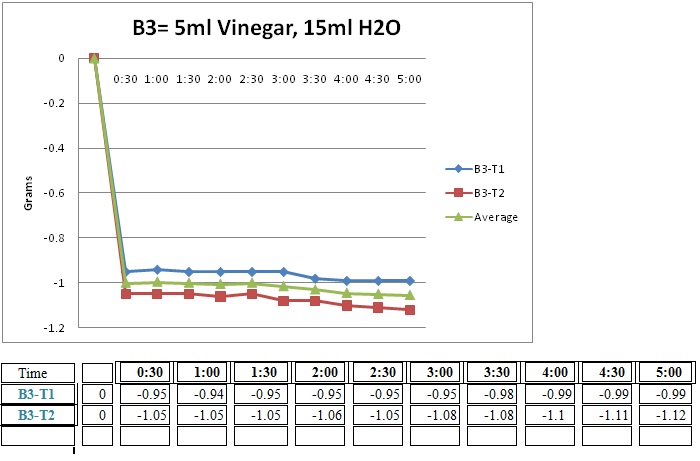
B3= 5ml Vinegar, 15ml H2O
B= Beaker B1= Beaker 1 etc.. T1= Trial 1 T2= Trial 2
This shows the amount it decreased
The initial reaction was all that took place. Because there was so little vinegar there wasn’t so much of a reaction. It goes down by a gram and then it went down by very little. Both of the trials gave very similar results and this was just as I expected.
My hypothesis was more or less correct. The results showed that it went down by 1.7 grams. I was able to get such a sound hypothesis from the background information. It show how the neutralization reaction is more when there is a greater concentrate of vinegar. I can conclude that the substance with more vinegar would always have a greater neutralization reaction. However B2 results for one of them came very close to B1.
1. http://en.wikipedia.org/wiki/Vitamin_C
2. http://www.whfoods.com/genpage.php?tname=nutrient&dbid=109
3. http://www.lenntech.com/fruit-vegetable-vitamin-content.htm
4. http://www.google.com.my/search?q=vitamin+c