





Published on Apr 02, 2024
In this experiment we will determine the amount of vitamin C (ascorbic acid) in different fruit juices by titration of the juice with a solution of iodine. The iodine reacts rapidly with the vitamin C. If you have a juice you would like to analyze, bring about 125 ml to lab. Compare at least two juices.
Vitamin C or known as L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate which is an ion of ascorbic acid acts as an antioxidant by protecting the body against oxidative stress. It is also a cofactor in at least eight enzymatic reactions . Vitamin C also aids in detoxification and in improving ferum absorption. Other that that, vitamin C also ensure the maintenance of cartilage, bone, denin and healthy blood vessel.. Humans and rodents cannot synthesis vitamin C but most of other animals have the ability to synthesis vitamin C.
Thus intake of vitamin C ensure healthy lifestyle. Since vitamin C is a water soluble vitamin, since it cannot be stored in human body. The uses and recommended daily intake of vitamin C are matters of ongoing debate, with RDI ranging from 45 to 95 mg/day. Vitamin C is most present in the liver and least present in the muscle. Since muscle provides the majority of meat consumed in the western human diet, animal products are not a reliable source of the vitamin. Vitamin C is present in mother's milk but, not present in raw cow's milk. All excess vitamin C is disposed of through the urinary system.
Apricots, apples, banana, blackberries, cherries, kiwi, grapes, lemon, lime ,mango, lychee, melon, orange, peach, pear, pineapple, plum, pumpkin, raspberries, strawberry, tomato and watermelon are some of fruits that contain high content of vitamin C. In the other hand, vegetables like artichoke, asparagus, avocado, broccoli, cabbage, corn, paprika, mushroom and spinach have vast content of vitamin C.
Which type of juice provide the most vitamin C?
The higher the volume of fruit juices needed to decolourise DCPIP solution, the lower the vitamin C content in the fruit juice. Fresh lime juice has the highest content of Vitamin C among the fresh juices and orange cartoon juice has the highest content of Vitamin C among carton juices.
Test tubes, 0.5ml syringe,10ml syringe, beaker, mortar and pestle
1000mg vitamin C tablets, 1% dichlorophenolindophenol solution (DCPIP), freshly squeezed lime, lemon and orange juices, 100ml distilled water, lime, lemon and orange carton juice.
1. A full vitamin C weighing 1g was crushed into fine powder with mortar and pestle.
2. The powder was then dissolved in 100ml of distilled water to form 1g/100ml ascorbic acid.
3. Steps 1 and 2 were repeated thrice to get average volume of vitamin C needed to decolourise DCPIP solution.
4. Steps 1 until 3 were repeated by using ¾ tablet, ½ tablet, ¼ tablet and ⅛ tablet of vitamin C to produce 0.75g/100ml , 0.5g/100ml , 0.25g/100ml and 0.125g/100ml respectively.
1. 0.5ml of 1.0% DCPIP solution was measured using 0.5ml syringe and placed into a test tube.
2. Then, 1ml of 1g/100ml ascorbic acid was measured using a syringe.
3. The ascorbic acid was titrated drop by drop into test tube containing DCPIP solution.
4. Ascorbic acid was added with extra care, drop by drop until the blue color of DCPIP solution turn colourless.
5. The volume of 1g/100ml of ascorbic acid was measured and recorded.
6. Steps 1 to 5 were repeated using 0.75g/100ml , 0.5g/100ml , 0.25g/100ml and 0.125g/100ml respectively to obtain respective measurement.
7. A standard curve was plotted base on the results.
1. 0.5ml of 1.0%DCPIP solution was measured and placed into a test tube.
2. Then, 5ml of freshly squeezed orange juice was measured using a syringe.
3. The needle of the syringe was placed into the DCPIP solution.
4. The freshly squeezed orange juice was added drop by drop to the DCPIP solution. The mixture gently stirred with the needle of the syringe. The juice is continuously added with extra care until the DCPIP solution is decolourised. The step is repeated thrice.
5. The volume of fruit juice needed to decolourise the DCPIP solution was recorded.
6. If more than 5 cmᵌ of fruit juice needed to decolourise the DCPIP solution, then one control colour was set up. With another test tube containing DCPIP solution, fruit juice was added until the colour changes exactly the same. The volume of fruit juices needed was recorded.
7. Steps 1 until 5 were repeated by replacing freshly squeezed orange juice with freshly squeezed lemon juice and freshly squeezed lime juice. The data was recorded.
8. Steps 1 until 5 were repeated by replacing freshly squeezed orange juice with orange carton juice, lemon carton juice and lime carton juice. The data was tabulated.
9. The concentration of each group of juice and each of the juice was calculated by using following formula :

10. The readings obtained were tabulated in the table.
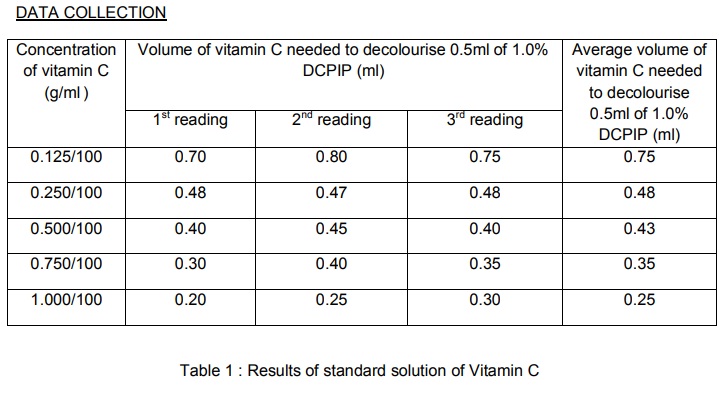
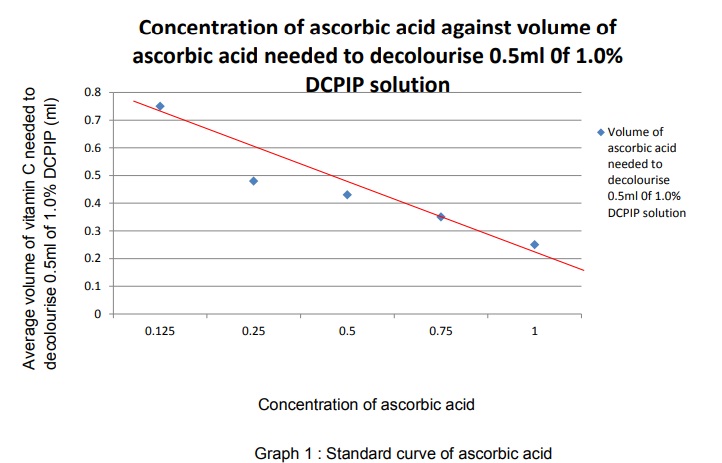
Every fruit group has different concentration of vitamin C. the smaller the volume of fruit juice needed to decolourise DCPIP solution, the higher the content of vitamin C in fruit juice. Fresh juice contain more vitamin C content than carton fruit juices. Fresh lime juice has the highest vitamin C content and lime has the lowest vitamin C content among fresh fruit juices while for carton fruit juices, orange has highest and lime has the lowest content of vitamin C. the hypothesis is accepted.
1. http://en.wikipedia.org/wiki/Vitamin_C
2. http://www.whfoods.com/genpage.php?tname=nutrient&dbid=109
3. http://www.lenntech.com/fruit-vegetable-vitamin-content.htm
4. http://www.google.com.my/search?q=vitamin+c