





Published on Apr 02, 2024
To Study of the Effect of Metal Coupling on the Rate of Corrosions
Corrosion is the gradual destruction of material, usually metals, by chemical reaction with its environment. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of ironoxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term degradation is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases.
Many structural alloys corrode merely from exposure to moisture in the air, but the process can be strongly affected by exposure to certain substances. Corrosion can be concentrated locally to form a pit or crack, or it can extend across a wide area more or less uniformly corroding the surface. Because corrosion is a diffusion-controlled process, it occurs on exposed surfaces. As a result, methods to reduce the activity of the exposed surface, such as passivation and chromate conversion, can increase a material's corrosion resistance. However, some corrosion mechanisms are less visible and less predictable.
Beakers-15, Iron sheets of 2# size-6, Aluminium rods of 2# size-6, Brass rods of 2# size-6, Zinc sheets of 2# size-6, Measuring cylinders, Chemical Balance, WeightBox.
Hydrochloric acid and Sodium hydroxide
(i) Mix 9 ml. of conc. HCl with 241 ml. of water to form 250 ml. of solution.
(ii) Take this solution in seven different beakers.
(iii) Mark each beaker serially from 1 to 7.
(iv) Take the weights of three iron sheets, three aluminium rods, three brass rods and three Zinc sheets
(v) Now keep iron sheets, aluminium rods, zinc sheets and brass rod in separate beakers
(vi) Then take iron + brass, iron + aluminium, iron + zinc, aluminium + zinc andbrass + zinc and keep them in different beakers.
(vii) Allow the reactions to occur for 24 hours.
(viii) Note the maximum and minimum temperatures.
(ix) Now at the end of reaction take out the metals and keep them in sun forsometime so that they get dried up quickly
(x) Take the weights of each specimen and note the difference.
(xi) Similarly repeat 1,2,3,4,5,6,7 and 8 steps in a basic solution.
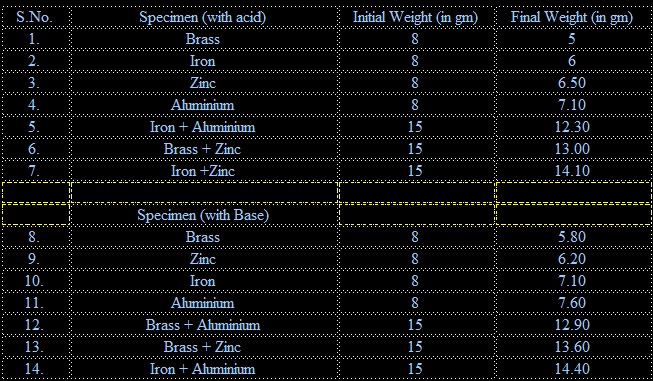
1. The rate of corrosion observed in acidic medium or the mass consumed during the Corrosion is in the decreasing order from brass to aluminium. Brass has the highest Corrosion rate while aluminium has the least corrosion rate.
Brass > Iron > Zinc > Aluminium
2. When coupling of these metals was done each couple showed some difference in their corrosion with respect to each metal kept alone. Iron + Aluminium couple has the highest rate of corrosion while iron +Zinc couple has the lowest rate of corrosion. Rate of corrosion of each couple is in the order of
Iron + Aluminium > Brass + Zinc> Iron + Zinc
3. Rate of corrosion in basic medium is in the decreasing order from Brass to Aluminium. The order of rate of corrosion is as below:
Brass > Zinc >Iron > Aluminium
4. When these metals were coupled the rate of corrosion was in the decreasing order from
Brass+ Aluminium > Brass + Zinc > Iron + Aluminium
5. Temperature and time of reaction were constant i.e., temperature was 21° C and time of reaction was 24 hours.
• Corrosion is a serious problem of some metals like iron, zinc, aluminium and alloys like brass which are commonly used in day to day life.
• Apart from reducing the life of articles made up of these metals or alloys the chemical substances formed out of corrosion have serious public health problems.
• Replacement of machines or their parts and many other articles in industrial and public dealing lead to huge expenditure.
• Hence, how to reduce or avoid corrosion of articles made up of metals or alloys has been a major subject of study in the field of chemistry and electro-chemistry.
• The study of the rate of corrosion of different metals or alloys showed gradual decrease in their masses in acidic medium. The decrease is in the order of brass, iron, zinc, aluminium.
• The present experiments are in full agreement with the well known electro-chemical reaction. Some of the typical reactions as occur with iron are illustrated.
NCERT Chemistry- XII
Comprehensive Practical Chemistry- XII
www..scribd..com