





Published on Apr 02, 2024
In this project we shall investigate the various factors that have already been discussed such as nature of the liquid, surface area of liquid & temperature and find their co relation with the rate of evaporation of different liquids. Each liquid surface has a specific heat of vaporization that means the quantity of heat energy required to change exactly one gram of liquid into its vapor at its boiling point.
In this project its Compared The Rate Of Evaporisation Of Water, acetone & diethylether
For molecules of a liquid to evaporate, they must be located near the surface, be moving in the proper direction, and have sufficient kinetic energy to overcome liquid-phase intermolecular forces.[1] Only a small proportion of the molecules meet these criteria, so the rate of evaporation is limited. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperature. As the faster-moving molecules escape, the remaining molecules have lower average kinetic energy, and the temperature of the liquid thus decreases. This phenomenon is also called evaporative cooling. This is why evaporating sweat cools the human body. Evaporation also tends to proceed more quickly with higher flow rates between the gaseous and liquid phase and in liquids with higher vapor pressure. For example, laundry on a clothes line will dry (by evaporation) more rapidly on a windy day than on a still day. Three key parts to evaporation are heat, humidity and air movement
If evaporation takes place in a closed vessel, the escaping molecules accumulate as a vapor above the liquid. Many of the molecules return to the liquid, with returning molecules becoming more frequent as the density and pressure of the vapor increases. When the process of escape and return reaches an equilibrium, [1] the vapor is said to be "saturated," and no further change in either vapor pressure and density or liquid temperature will occur. For a system consisting of vapor and liquid of a pure substance, this equilibrium state is directly related to the vapor pressure of the substance, as given by the Clausius-Clapeyron relation:
Where PI, P2 are the vapor pressures at temperatures Tl, T2 respectively, ?Hvap is the enthalpy of vaporization, and R is the universal gas constant. The rate of evaporation in an open system is related to the vaj or pressure found in a closed system. If a liquid is heated, when the vapor pressure reaches the ambient pressure the liquid will boil.
The ability for a molecule of a liquid to evaporate is largely based on the amount of kinetic energy an individual particle may possess. Even at lower temperatures, individual molecules of a liquid can potentially evaporate if they have more than the minimum amount of kinetic energy required for vaporization
To compare the rate of evaporation of water, acetone and di ethyl ether.
Three weight bottles, lOmL pipettes, stopwatches etc. ...
1 . Clean and dry the three weight bottles and label the A, B, and C.
2. Pipette out lOmL of water, acetone and diethyl ether onto the three weight bottles A, B and C.
3. Cover the three bottles with a stopper. ;
4. Weigh each of the bottles and record their weights.
5. Remove the stopper from all the three bottles and start the stopwatch.
6. Let the bottles remain exposed for thirty minutes. Now cover each of the bottles again and weigh them again
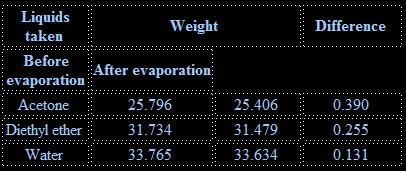
Rate of evaporation of diethyl ether is greater than that of acetone and that of water
To study the effects of surface area on the rate of evaporation of diethyl ether and acetone.
3 petridishes of 2.5,5,10 cm with cover
10 ml pipettes, stop watch.
1 . Clean and dry the petridishes and mark them A, B, C.
2. Pipette out 10 ml diethyl ether in each of the petridishes; A, B, C and cover them immediately.
3. Uncover all the petridishes simultaneously and start the stopwatch.
4. Note the time when diethyl ether evaporates completely from each petridish.
5. Repeat the experiment with acetone.
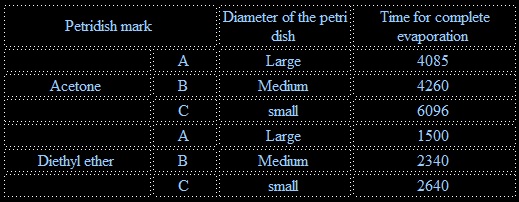
It is observed that maximum evaporastion occurs in the petridish with largest diameter is followed by the smaller and the smallest. It is therefore concluded the rate of evaporation increases wih increase in surface area
From the experiments, it is clear that The Rate of evaporation depends upon
The nature of the liquid.
The surface area of the liquid.
Temperature.
Flow of air current over the liquid surface
• Practical manual in chemistry V.K.Bhandari
• www.scienceprojects.com
• Encarta Encyclopedia
• Wikipedia.com the open source encyclopedia on the net