





Published on Apr 02, 2024
Aim is To Analyze a Sample of Brass Qualitatively.
Brass is an alloy of copper and zinc. Small amount of iron and lead are also present. This dissolves in 50%nitric acid and generates the ions of the constituting metals in solutions. Presence of these ions in the solution can be conformed by qualitative inorganic analysis scheme.
An alloy is a homogeneous mixture of two or more metals or metals and non-metals. In other words a solid solution of two or more metals or a metal and a non-metal. It is prepared by first melting the main metal, and then, dissolving the other elements in it in a definite proportion. It is then cooled to the room temperature. If one of the metals is mercury, then the alloy is known as amalgam. The electrical conductivity of an alloy is less than that of pure metals.
More than ten thousands alloys have been prepared so far and about hundred of them are in common use, for example, stainless steel, brass, bronze, duralumin, soldering metal, gun metal, etc. So large numbers of alloys have been prepared because the properties of the parent metals constituting the alloy are modified a lot by alloy formation. For example, pure copper metal is soft and ductile, but when it is alloyed with small amount of zinc, becomes hard.
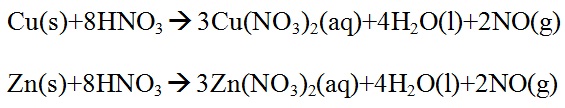
Thus, alloys are made to improve the following properties of metals:
• For increasing hardness
• For increasing tensile strength
• For increasing chemical resistance
• For lowering melting point
• For modifying colour
Alloys are prepared from the metals generally by fusion technique. That is, metals are converted first to molten state, mixed well and then allowed to solidify again. A number of methods, instrumental as well as chemical, are known for finding out the constituents of an alloy. In chemical method, first a solution of the alloy is being prepared, and then presence of various constituents can be tested either by applying spot test i.e., making use of different organic reagents or by applying regular qualitative inorganic analysis scheme
Test tubes, test-tube stand, beakers, funnel, test-tube holder, sand paper, filter paper
Chemicals: Common laboratory reagents
(i) Clean a piece of brass with sand paper. Wash it with water. Cut it into small pieces and place the pieces in a clean beaker.
(ii) Add to it about 10ml of 50% nitric acid. Heat the contents in a fume-closet until the brass pieces dissolve. Concentrate the solution to a pasty mass.
(iii) Dissolve the residue in about 10ml of hot distilled water and filter if there is any turbidity. The solution so obtained will be the original solution for inorganic analysis.
(iv) Follow the following procedure for detection of various cations present in the original solution.
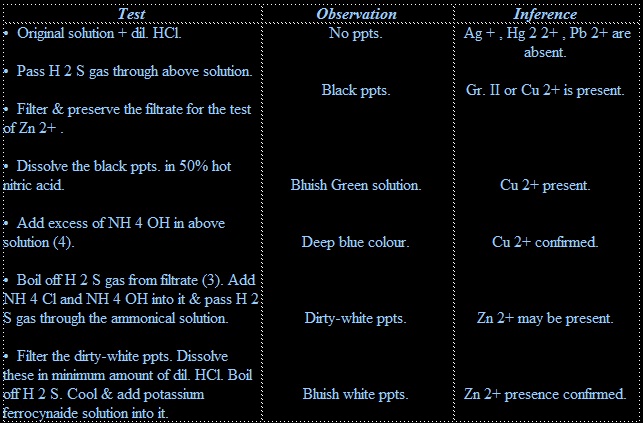
• Brass contains copper and zinc metals.