





Published on Apr 02, 2024
A paper battery is a flexible, ultra-thin energy storage and production device formed by combining carbon nanotube s with a conventional sheet of cellulose-based paper. A paper battery acts as both a high-energy battery and supercapacitor , combining two components that are separate in traditional electronics .
This combination allows the battery to provide both long-term, steady power production and bursts of energy. Non-toxic, flexible paper batteries have the potential to power the next generation of electronics, medical devices and hybrid vehicles, allowing for radical new designs and medical technologies.
Paper batteries may be folded, cut or otherwise shaped for different applications without any loss of integrity or efficiency . Cutting one in half halves its energy production. Stacking them multiplies power output. Early prototypes of the device are able to produce 2.5 volt s of electricity from a sample the size of a postage stamp
They have produced a sample slightly larger than a postage stamp that can store enough energy to illuminate a small light bulb. But the ambition is to produce reams of paper that could one day power a car.
Professor Robert Linhardt, of the Rensselaer Polytechnic Institute, said the paper battery was a glimpse into the future of power storage. The team behind the versatile paper, which stores energy like a conventional battery, says it can also double as a capacitor capable of releasing sudden energy bursts for high-power applications.
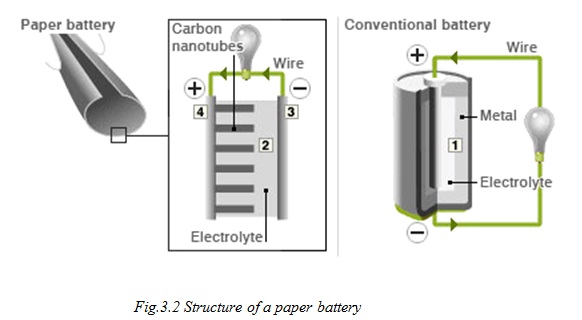
While a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient.
The research appears in the Proceedings of the National Academy of Sciences (PNAS).
"Think of all the disadvantages of an old TV set with tubes," said Professor Linhardt, from the New York-based institute, who co-authored a report into the technology.
"The warm up time, power loss, component malfunction; you don't get those problems with integrated devices. When you transfer power from one component to another you lose energy. But you lose less energy in an integrated device." The battery contains carbon nanotubes, each about one millionth of a centimetre thick, which act as an electrode. The nanotubes are embedded in a sheet of paper soaked in ionic liquid electrolytes, which conduct the electricity. The flexible battery can function even if it is rolled up, folded or cut. Although the power output is currently modest, Professor Linhardt said that increasing the output should be easy.
A very brief explanation has been provided.
• Cathode: Carbon Nanotube (CNT)
• Anode: Lithium metal (Li+)
• Electrolyte: All electrolytes (incl. bio electrolytes like blood, sweat and urine)
• Separator: Paper (Cellulose)
The process of construction can be understood in the following steps:
• Firstly, a common Xerox paper of desired shape and size is taken.
• Next, by conformal coating using a simple Mayer rod method, the specially formulated ink with suitable substrates (known as CNT ink) is spread over the paper sample.
• The strong capillary force in paper enables high contacting surface area between the paper and nanotubes after the solvent is absorbed and dried out in an oven.
• A thin lithium film is laminated over the exposed cellulose surface which completes our paper battery. This paper battery is then connected to the aluminum current collectors which connect it to the external load.
• The working of a paper battery is similar to an electrochemical battery except with the constructional differences.
The paper battery is designed to use a paper-thin sheet of cellulose (which is the major constituent of regular paper, among other things) infused with aligned carbon nanotubes. The nanotubes act as electrodes, allowing the storage devices to conduct electricity. The battery will currently provide a low, steady power output, as well as a supercapacitor’s quick burst of energy. While a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient and lighter.
The creation of this unique nanocomposite paper drew from a diverse pool of disciplines, requiring expertise in materials science, energy storage, and chemistry. In August 2007, a research team at Rensselaer Polytechnic Institute (led by Drs. Robert Linhardt, the Ann and John H. Broadbent Senior Constellation Professor of Biocatalysis and Metabolic Engineering at Rensselaer; Pulickel M. Ajayan, professor of materials science and engineering; and Omkaram Nalamasu, professor of chemistry with a joint appointment in materials science and engineering) developed the paper battery.
Senior research specialist Victor Pushparaj, along with postdoctoral research associates Shaijumon M. Manikoth, Ashavani Kumar, and Saravanababu Murugesan, were co-authors and lead researchers of the project. Other co-authors include research associate Lijie Ci and Rensselaer Nanotechnology Center Laboratory Manager Robert Vajtai. The researchers used ionic liquid, essentially a liquid salt, as the battery’s electrolyte. The use of ionic liquid, which contains no water, means there’s nothing in the batteries to freeze or evaporate. “This lack of water allows the paper energy storage devices to withstand extreme temperatures,” Kumar said. It gives the battery the ability to function in temperatures up to 300 degrees Fahrenheit and down to 100 below zero. The use of ionic liquid also makes the battery extremely biocompatible; the team printed paper batteries without adding any electrolytes, and demonstrated that naturally occurring electrolytes in human sweat, blood, and urine can be used to activate the battery device. According to Pushparaj “It’s a way to power a small device such as a pacemaker without introducing any harsh chemicals – such as the kind that are typically found in batteries — into the body.
The use of carbon nanotubes gives the paper battery extreme flexibility; the sheets can be rolled, twisted, folded, or cut into numerous shapes with no loss of integrity or efficiency, or stacked, like printer paper (or a Voltaic pile), to boost total output. As well, they can be made in a variety of sizes, from postage stamp to broadsheet. “It’s essentially a regular piece of paper, but it’s made in a very intelligent way,” said Linhardt, “We’re not putting pieces together — it’s a single, integrated device,” he said. “The components are molecularly attached to each other: the carbon nanotube print is embedded in the paper, and the electrolyte is soaked into the paper. The end result is a device that looks, feels, and weighs the same as paper.”
The paper-like quality of the battery combined with the structure of the nanotubes embedded within gives them their light weight and low cost, making them attractive for portable electronics, aircraft, automobiles, and toys (such as model aircraft), while their ability to use electrolytes in blood make them potentially useful for medical devices such as pacemakers. The medical uses are particularly attractive because they do not contain any toxic materials and can be biodegradable; a major drawback of chemical cells However, Professor Sperling cautions that commercial applications may be a long way away, because nanotubes are still relatively expensive to fabricate. Currently they are making devices a few inches in size. In order to be commercially viable, they would like to be able to make them newspaper size; a size which, taken all together would be powerful enough to power a car.
A paper battery is a battery engineered to use a paper-thin sheet of cellulose (which is the major constituent of regular paper, among other things) infused with aligned carbon nanotubes.[1] The nanotubes act as electrodes; allowing the storage devices to conduct electricity. The battery, which functions as both a lithium-ion battery and a supercapacitor, can provide a long, steady power output comparable to a conventional battery, as well as a supercapacitor’s quick burst of high energy -- and while a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |