





Published on Apr 02, 2024
Nuclear batteries use the incredible amount of energy released naturally by tiny bits of radio active material without any fission or fusion taking place inside the battery. These devices use thin radioactive films that pack in energy at densities thousands of times greater than those of lithium-ion batteries. Because of the high energy density nuclear batteries are extremely small in size.
Considering the small size and shape of the battery the scientists who developed that battery fancifully call it as "DAINTIEST DYNAMO". The word 'dainty' means pretty. Scientists have developed two types of micro nuclear batteries. One is junction type battery and the other is self-reciprocating cantilever. The operations of both are explained below one by one.
As the Ni-63 decays it emits beta particles, which are high-energy electrons that spontaneously fly out of the radioisotope's unstable nucleus. The emitted beta particles ionized the diode's atoms, exciting unpaired electrons and holes that are separated at the vicinity of the p-n interface. These separated electrons and holes streamed away form the junction, producing current. It has been found that beta particles with energies below 250KeV do not cause substantial damage in Si [4] [5].
The maximum and average energies (66.9KeV and 17.4KeV respectively) of the beta particles emitted by Ni-63 are well below the threshold energy, where damage is observing silicon. The long half-life period (100 years) makes Ni-63 very attractive for remote long life applications such as power of spacecraft instrumentation. In addition, the emitted beta particles of Ni-63 travel a maximum of 21 micrometer in silicon before disintegrating; if the particles were more energetic they would travel longer distances, thus escaping. These entire things make Ni-63 ideally suitable in nuclear batteries.
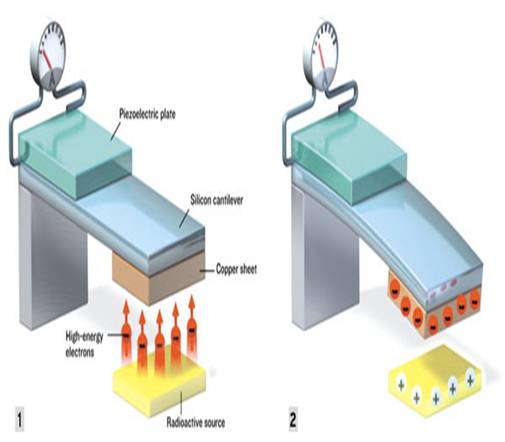
First the beta particles, which are high-energy electrons, fly spontaneously from the radioactive source. These electrons get collected on the copper sheet. Copper sheet becomes negatively charged. Thus an electrostatic force of attraction is established between the silicon cantilever and radioactive source. Due to this force the cantilever bends down.
The piece of piezoelectric material bonded to the top of the silicon cantilever bends along with it. The mechanical stresses of the bend unbalances the charge distribution inside the piezoelectric crystal structure, producing a voltage in electrodes attached to the top and bottom of the crystal. After a brief period - whose length depends on the shape and material of the cantilever and the initial size of the gap- the cantilever come close enough to the source to discharge the accumulated electrons by direct contact. The discharge can also take place through tunneling or gas breakdown.
At that moment, electrons flow back to the source, and the electrostatic attractive force vanishes. The cantilever then springs back and oscillates like a diving board after a diver jumps, and the recurring mechanical deformation of the piezoelectric plate produces a series of electric pulses. How a nuclear micro generator converts radioactivity into electricity
• Beta particles (high-energy electrons) fly spontaneously from the radioactive source and hit the copper sheet, where they accumulate.
• Electrostatic attraction between the copper sheet and the radioactive source bends the silicon cantilever and the piezoelectric plate on top of it.
• When the cantilever bends to the point where the copper sheet touches the radioactive source, the electrons flow back to it, and the attractive force ceases.
• The cantilever then oscillates, and the mechanical stress in the piezoelectric plate creates an imbalance in its charge distribution, resulting in an electric current.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |