





Published on Nov 30, 2023
A trend of significant increase in the municipal solid waste generation has been recorded worldwide. This hasbeen found due to over population growth rate, industrialization, urbanization and economic growth.Most of the countries have adopted sanitary landfilling as the best method for disposal of their MSW. One of the major pollution problems caused by the sanitary landfill is landfill leachate, which is generated as consequences of infiltration of water into landfills and squeezing of the waste due to self-weight. Landfill leachate contains a wide variety of recalcitrant compounds such as organic matter, heavy metals and inorganic salts, which makes it quite difficult to treat using conventional ways of treatment due to its high chemical stability and/or low biodegradability.In recent years, a great deal of attention has been focused on to the application of nanosized metal oxides to treat heavy metals, organic and inorganic matter by nanosized titanium oxides, ferric oxides, manganese oxides, aluminium oxides and magnesium oxides as adsorbents and photocatalysts. The utilization of TiO2 nanomaterial as an adsorbent and photocatalytic has received much attention due to its chemical stability, non-toxic and photostable.
In the present study, the photocatalytic degradation of synthetic leachate was investigated in natural sunlight by using TiO2 as Nanomaterial. The parabolic trough collector is used as solar photoreactor, which can efficiently bring solar photons and chemical reagents into contact with the photocatalyst. The characterization of TiO2is conducted by X-Ray Diffraction (XRD) and Scanning Electron Microscope (SEM).The influences of various parameters such as photocatalytic dosage and contact time are studied on leachate removal efficiency. The result indicates thatXRD and SEM confirm that the selected photocatalyst TiO2 is an anatase with spherical in shape. The crystallite size is approximately 19nm and specific surface area of 120.32 m2/gm. The recipe used for the preparation of synthetic leachate have a similar composition of real landfill leachate. The influencing parameters dosage and contact time are able to remove the maximum percentage of organic and inorganic compounds from synthetic leachate.The average removal efficiency of lead is 97.82% in alkaline pH 9 with contact time 80 minutes and dosage of 0.3g/l
Keywords : Solid Waste, Sanitary Landfill, synthetic leachate, X-Ray Diffraction, Scanning Electron Microscope
Solid waste is the unwanted or useless solid materials generated from human activities in residential, industrial or commercial areas. It may be categorised in three ways. Based on, origin(domestic, industrial, commercial, construction or institutional), contents (organic material, glass, metal, plastic paper etc) and hazard potential (toxic, non-toxin, flammable, radioactive, infectious etc).
Municipal solid waste (MSW) consists of household waste, construction and demolition debris, sanitation residue, and waste from streets, generated mainly from residential and commercial complexes. In metro cities in India, an individual produces an average of 0.8 kg/ waste/ person daily. The total municipal solid waste (MSW) generated in urban India has been estimated at 68.8 million tons per year (TPY). The average collection efficiency of MSW ranges from 22% to 60%.MSW typically contains 51% organic waste, 17% recyclables, 11% hazardous and 21% inert waste.
The remaining 40% of MSW lies littered in the city/town and finds its way to nearby drains and water bodies, causing choking as well as pollution of surface water. The collected MSW will be disposed by various methods such as sanitary land filling, incineration, open burning, composting, and dumping into the sea. One of the most commonly used MSW disposal method all over the world is sanitary landfill.
Sanitary landfills are a method of waste disposal where the waste is buried either underground or in large piles. This method of waste disposal is controlled and monitored very closely. The sanitary landfillare classified into three types, Mechanized sanitary landfill, Semi-mechanised sanitary landfill and manual sanitary landfill.
For sanitary landfills, the process starts by digging a large hole in the ground that is then lined with thick plastic (normally 2-4 feet thick) and a layer of impervious clay. The bottom of the landfill is also lined with a network of plumbing that functions as a collection system for any liquids. There will be two types of wastes are generated from the sanitary landfill those are of methane gas and leachat.Leachate is the term used to describe liquids that leach or leak from the landfill.
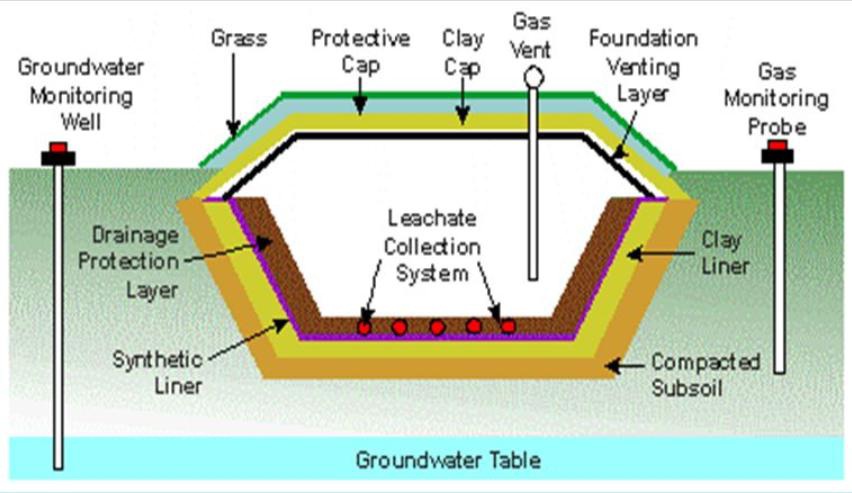
Leachate may be defined as liquid that has percolated through solid waste and has extracted dissolved or suspended materials from it. In most landfills, the liquid portion of the leachate is composed of the liquid produced from the decomposition of wastes and liquid that as entered the landfill from external sources such as surface drainage, rainfall, ground water and water from underground springs. The black liquid contains organic and inorganic chemicals, heavy metals as well as pathogens; it can pollute the ground water and represents the health risk. Its composition varies a lot, both from time to time and from site to site so that it is difficult to treat the liquid in the right way.
Leachates from landfill are generated by a number of factors, such as:
Infiltration of ground water;
Infiltration of leachate into the ground (a potential pollution of the ground water may occur);
Rainfall (precipitation);
Water from the deposited waste, mainly due to the static pressure;
Evaporation from the site.
Leachate is highly complex and polluted waste water that is produced by the introduction of percolation water through the body of landfill treatment. Leachate treatment is essential as it could threaten the surrounding ecosystem when discharge as it is and when it mixes with groundwater. There are different methods of leachate treatment such as coagulation-Flocculation, chemical precipitation, flotation, activated carbon adsorption, ion exchange chemical oxidation and advanced oxidation process and nanomaterial. Heavy metals appear in the leachate due to batteries,consumers electronics, ceramics, light bulbs, house dust and paint chips,. Concentration of heavy metals in a leachate is generally higher at earlier stages because of higher metal solubility as a result of low pH caused by production of organic acids. It is now recognised that most trace elements are readily fixed and accumulate in soils, and providing threat to human health and environment.
Photocatalysis is the acceleration of a photoreaction in the presence of a catalyst.A photocatalyst is defined as a substance which is activated by adsorbing a photon and is capable of accelerating a reaction without being consumed. There are two types of photocatalysis they are homogeneousphotocatalysis and heterogeneousphotocatalysis.
In homogeneous photocatalysis, the reactants and photocatalysts exits in the same phase. The most commonly used homogeneous photocatalysts include ozone and photo-Fenton systems. The efficiency of Fenton type processes is influenced by several operating parameters like concentration of hydrogen peroxide, pH and intensity of UV. The main advantage of this process is the ability of using sunlight with light sensitivity up to 450nm, thus avoiding the high costs of UV lamps and electrical energy. These reactions have been proven more efficient than the other photocatalysis but the disadvantages of the processes are the low pH valueswhich are required, since iron precipitates at higher pH values and the fact that iron has to be removed after treatment.
Heterogeneous photocatalysis has the catalyst in different phase from the reactants. The most commonly used heterogeneous photocatalysts are transition metal oxide and semiconductors. Semiconducting oxide photocatalyst have been increasingly focused in recent years due to their potential applications in solar energy conversion and environmental purification. Semiconductor heterogeneous photocatalysis has enormous potential to treat organic contaminants in water and air, this process is known as advanced oxidation process (AOP) and is suitable for the oxidation of wide range of organic compounds. Among AOPs, heterogeneous photocatalysis have been proven to be of interest due to its efficiency in degrading recalcitrant organic compounds.
The several semiconductors Tio2, ZnO, Fe2O3, CdS and ZnS can act as photocatalysts but TiO2 has been most commonly used due to its ability to break down organic pollutants and even achieve complete mineralization. Photocatalytic and hydrophilic properties of TiO2 makes it close to an ideal catalyst due to its high reactivity, less toxic, chemical stability and lower costs.
The main aim of this work is to study the performance and suitability of semiconductor TiO2 nanomaterials in treatment of heavy metals and organic compounds in the landfill leachate. In this context objectives are as listed below.
1. Characterization study of TiO2nanomaterial, such as particle size, specific surface area, and surface morphology.
2. Preparation of synthetic leachate in mark with young landfill leachate.
3. To study the influencing parameters on photocatalytic such as dosage and contact time
4. To determine the photocatalytic activity of titanium dioxide in removal of heavy metal lead.
5. To determine photocatalytic activity of titanium dioxide in removal of other parameters of leachate such as COD, nitrate, sulphates.
The Scanning Electron Microscope (SEM) and X-Ray powder diffraction (XRD) is used to study particle size, specific surface area and morphology of TiO2 Nanomaterials. Tests are carried out at Shivaji University Kolhapur. The titanium dioxide (TiO2) used was supplied from Sisco Research Laboratories Pvt. Ltd.
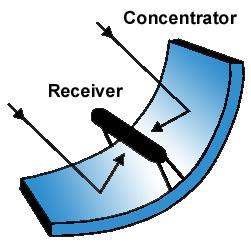
After conducting more research on solar energy and solar collection, the decision was made to attempt to build a parabolic trough solar concentrator. In parabolic concentrator all the incoming rays from a light source are reflected back to the focal point of the parabola.Parabola is built by eccentricity method. The photoreactor used was a transparent borosilicate glass tube with 3 cm internal diameter, 20.4 cm length, mounted on a parabolic collector of aperture length 38 cm and aperture width 18.2 cm (Fig. 3.1). The parabolic collector is coated with Aluminium foil to bring about 100% reflection of sunlight during photocatalysis. The photoreactor used for the study will be prepared with borosilicate glass tube with 38mm internal diameter, 1.8m length, mounted on a parabolic trough reflector of aperture length 172cm and aperture width 57.75cm.
COD is a measure of total quantity of oxygen required for oxidation of nearly all oxygen compounds in waste water, by the action of strong oxidising agent.
Reagents used:
Standard potassiumdichromate (0.25 N), COD reagent, Standard ferrous ammonium sulphate (0.1 N), Mercuric sulphate, Ferroin indicator.
1. Place 0.4 gm HgSO4 in a reflux flask.
2. Add 20 ml sample or an aliquot of sample diluted to 20ml. Mix well.
3. Add glass beads followed by 10ml standard potassium dichromate.
4. Add 30ml COD reagent (while adding the reagent cool the flask). Mix well. If the colour turns green either take fresh sample or with a lesser aliquot.
5. Connect the flask to condenser and reflux for 2 hours.
6. Cool the flask to room temperature.
7. Add 4-5 drops of Ferroin indicator. Bluish green colour is observed.
8. Titrate this solution against 0.1 N Standard Ferrous Ammonium Sulphate till colour changes to wine red.
9. Note down the burette reading.
COD in mg/l = ((A−B)× Normality of titrant ×Equivalent weight of oxygen ×1000) / ml of sample titrated
Where, A= ml of titrant used for blank
B= ml of titrant used for sample
This method is suitable for screening samples that have low organic matter contents. The NO3 calibration curve follows Beer‟s law up to 11 mg/l. Measurements of UV absorption at 220nm enables rapid determination of NO3.
Stock nitrate solution, Standard nitrate solution.
1. Pipette 10, 20, 30, 40 ml (2, 4, 6, 8mg/l) of standard nitrate solution in 50ml Nessler‟s tubes/volumetric flask.
2. Switch on the instrument which is located of the left hand rear side of the instrument.
3. The display will show „Elico‟ *SL 210*.
4. Press ENTER, the display will show D2 lamp testing alignment in progress.
5. Press ENTER, the display will show base line scan. Then press YES.
6. Press ENTER, the display will show MENU.
7. Then the different modes are displayed. Select the required mode. Example, quantitative from the main MENU.
8. Press the „1‟ and select „Standard‟ option.
9. Keep the prepared samples and distilled water in reference point.
10. Press ENTER and feed the wave length value.
11. Press ENTER number of standards.
12. Press ENTER and select the concentration units Ex. Ppm.
13. Press ENTER and cuter values standard 1 concentration value. Similarly enter values for all standards.
14. Press ENTER and select mode of absorbance input Ex. Measure.
15. Press ENTER and enter number of samples.
16. Keep cuvette filled with reference and other cuvettes filled with standards in the cuvette holder drum.
17. Display will show auto zero option say always „NO‟.
18. The spectrophotometer will start reading the reference and all the standards.
19. Then the display will ask for samples. Remove the standards and place the samples in the cuvettes and press „ENTER‟.
20. Now the instrument will start reading the samples.
21. Now the display will show different option like 1.View, 2. Print, 3. Modes.
22. Press view to see readings.
23. The data option like standards and samples are displayed.
24. Press „ESCAPE‟ to come back to main menu.
25. Switch of the Instrument.
Analysis of TiO2 Photocatalytic characteristics
Particle size of Titanium dioxide adsorbent The XRD pattern of TiO2 Photocatalytic is shown in table 3. The results shows six peaks at 25.29, 37.92, 48.09, 53.86, 55.12, and 62.7. The 2θ peaks at 25.29˚ and 48.09˚ confirm its anatase structure according to JCPDS Card.
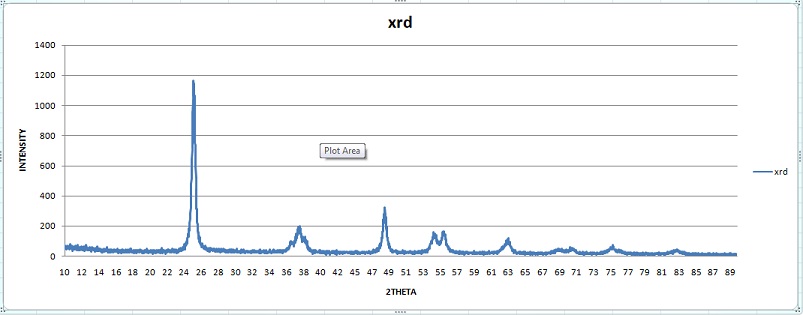
Average crystallite size is calculated by considering the 2θ peak values. The simplest and most widely used method for estimating the average crystallite size is from the full width half maximum (FWHM) of diffraction peak using Debye-Scherer formula
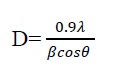
Where, λ is wave length of X-Ray (0.1540 nm), β is FWHM, θ is diffraction angle, and D is crystallite size. The average crystallite size obtained by the above equation is 19 nm. The intensity of XRD peaks of the sample reflects that the smaller the crystal size the broader the peak confirming small size crystallite
-The Graph 5 shows the reduction of COD in the synthetic leachate with 0.2g/l and 0.3g/l. As the catalysis dosage plays an important role in photocatalytic activity as the density of particle in the area of illumination increased. This was attributed to the increased availability of catalyst sites for the adsorption of the reactant molecules, better generation of reactive free radicals and their interaction.

In the present study, TiO2Photocatalytic nanomaterial is used for the removal of organic and inorganic compounds from synthetic leachate. Based on the present study following conclusions were drawn.
1. The TiO2 characteristics study, such as XRD and SEM results confirms that the selected photocatalyst TiO2 is an anatase with spherical in shape. The crystallite size is approximately 19nm and specific surface area of 120.32 m2/gm
2. The recipe used for the preparation of synthetic leachate have a similar composition of real landfill leachate.
3. To major two influencing parameters dosage and contact time, selected for the photocatalytic study has the ability to remove the maximum percentage of organic and inorganic compounds from synthetic leachate.
4. The removal efficiency of lead is maximum in alkaline pH 9 with contact time 80 minutes and dosage of 0.3g/l
5. The removal efficiency of COD, nitrate and sulphates were studied. COD removal efficiency found lesser due to the high pH value (alkaline) in synthetic leachate. The nitrate and sulphate removal efficiency were comparatively good by the TiO2photocatalyst