





Published on Apr 02, 2024
ELECTROLYTES - Are substances which allow the passage of electricity through their molten state or their aqueous solutions and allow chemical decomposition at the same time eg. Acids ,bases and salts.
ELECTROLYTIC CONDUCTION - There is a flow of electric current through the electrolytes with the motion of ions which are formed due to the dissociation of some molecules of the electrolyte.
OHM’S LAW – It states that under constant physical conditions such as temperature , pressure etc. the current flowing through the conductor is directly proportional to the potential difference across the two ends of the conductor.
Mathematically,
V = IR
Where,
V = potential difference across the ends of the conductor,
I = electric current through the conductor and,
R=resistance offered to the path of the electric current by the conductor
Using appropriate electrolytes and keeping the other factors constant , the concentration of a particular electrolyte(CuSo) was varied by intervals of 1%. Readings of resistances corresponding to different concentrations ranging from 1-10% were taken.
Expected result – the resistance of the electrolyte solution is expected to decrease with increase in concentration.
With increase in concentration , the number of ions due to dissociation of electrolyte increases. Since the rate of flow of charge is directly proportional to the number of ions, hence conductivity of the electrolyte increases and the resistance of the electrolyte decreases.
Key , rheostat , voltmeter ,ammeter , connecting wires , glass vessels , copper electrodes , measuring cylinders , physical balance , weights.
1. Apparatus is set up as shown , the vessel is cleaned and filled with 300ml of water.
2. 3gm of CuSo are weighed and dissolved in water , hence obtaining a concentration of 1%.
3. When the solution settles down and a constant temperature is attained, current is passed and three sets of readings of the voltmeter and ammeter are taken by adjusting the position of the rheostat after every set of readings.
4. The concentration of the electrolyte is increased to 2% by dissolving another 3gm of CuSo.
5. A set of three readings is taken by varying the resistance in the external circuit using the rheostat.
6. The concentration is stepped up each time by 1%in this manner up to 5%, and the readings for each set are noted.
7. The mean value of resistance is calculated in each case.
8. A graph is plotted , which shows the variation of resistance with concentration of the solution.
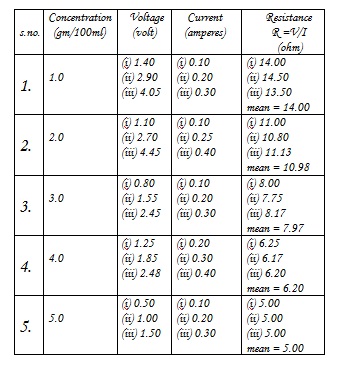
Result of the experiment shows that the resistance of an electrolyte is inversely proportional to its concentration. The conductivity of an electrolyte depends on the number of ions per unit volume i.e. concentration of ions. As the concentration of the solution is increased, the concentration of the ions and hence, the conductivity of the electrolyte also increases. Therefore resistance which is the reciprocal of conductance decreases with increase in concentration. This reasoning is supported by the results obtained for this experiment.
1. The electrodes used were thin plates of copper and they were always kept parallel to each other inside the electrolyte.
2. After each reading, the solution was stirred an allowed to come to rest before another reading was taken.
3. When variation of resistance due to one factor is measured, other factors affecting resistance must be kept constant.
1. While determining the resistance of electrolyte by using Cu electrodes, a small amount of the metal was deposited on the cathode. This would have caused a slight change in the concentration affecting the subsequent reading.
2. A small amount of oxygen is liberated at the anode which might have caused some polarization. These effects can be minimized by passing small currents for short time.
3. The concentration of the electrolyte could not be varied in a range other than between 1%-5% because beyond 5% concentration, it was not possible to take the readings and accumulation of impurities caused disturbances.