





Published on Apr 02, 2024
In this experiment you will synthesize aspirin and determine the yield or fraction of the theoretical amount which can be made. The purity of the product is confirmed by measuring its melting point range.
Aspirin is the common name for the compound acetylsalicylic acid, widely used as a fever reducer and as a pain killer. Salicylic acid, whose name comes from Salix, the willow family of plants, was derived from willow bark extracts. In folk medicine, willow bark teas were used as headache remedies and other tonics. Nowadays, salicylic acid is administered in the form of aspirin which is less irritating to the stomach than salicylic acid.
To prepare aspirin, salicylic acid is reacted with an excess of acetic anhydride. A small amount of a strong acid is used as a catalyst which speeds up the reaction. In this experiment, phosphoric acid will be used as the catalyst. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. The synthesis reaction of aspirin is shown below:
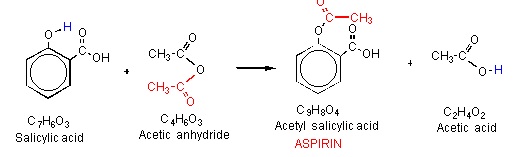
Since acetic acid is very soluble in water, it is easily separated from the aspirin product. The aspirin isolated in this step is the “crude product”. A “purified product” can be obtained through recrystallization of the crude product in hot ethanol. In this experiment, the crude product will be the desired product. The percent yield of the crude product will be determined for this reaction. The purity of the product will also be analyzed. The product will be analyzed by three different methods: melting point, titration, and spectroscopic assay. The melting point range of pure aspirin is 138-140 C and the melting point range of the salicylic acid starting material is 158-161 C. If impurities are present in your crude sample, the melting point range for your product will be lower than the range of pure aspirin. Also, your melting point range may be greater than 2 degrees. From the titration of your sample, the moles of acetylsalicylic acid present can be determined assuming that there is not a large percentage of an acid impurity present in your crude sample.
The spectroscopic analysis of aspirin will involve the complexing of iron(III) to the deprotonated form of salicylic acid (salicylate ion) to give a purple solution. Only the salicylate ion complexes to iron(III). Your aspirin product as well as a commercial aspirin tablet will be compared to a standard 0.15% ferricsalicylate solution. In the presence of moisture, aspirin may decompose (hydrolysis) into salicylic acid and acetic acid. This reaction is the reverse of the synthesis reaction. The maximum allowable amount of free salicylic acid in an aspirin sample is 0.15% salicylic acid.
This experiment uses salicylic acid, acetic anhydride and phosphoric acid. The salicylic acid and aspirin may cause irritation to your skin or eyes, but are basically not hazardous. An excess of these can be disposed of in the sink or if packaged, in the trash. If you spill some, wipe it up with a wet paper towel and throw the towel in the trash. The acetic anhydride and phosphoric acid can cause bad burns. Use them in the hood. Be sure to wear gloves and safety goggles when using these chemicals. Excess chemicals must be disposed of in the plastic tub of water. This will convert the acetic anhydride to vinegar and dilute the phosphoric acid. If you spill a lot of either of these, notify your instructor.
1. Weigh out 3.0 g of salicylic acid and place in a 250 ml Erlenmeyer flask.
2. Measure out 6.0 ml of acetic anhydride and add this to your flask. Be sure to do this in the hood and wear your goggles.
Don't let the acetic anhydride contact your skin and don't get the vapors in your eyes.
3. Carefully add 5 to 10 drops of 85% phosphoric acid, a catalyst, to the flask and swirl to mix everything thoroughly.
4. Still in the hood, heat the mixture for about 10 min. in a beaker of warm water (70-80 oC).
5. After heating, cautiously add 20 drops of distilled water.
6. Next add 20 ml of distilled water and cool in an ice bath. You can do this at your bench. If crystals do not appear, you can scratch the walls of the flask with a stirring rod to induce crystallization.
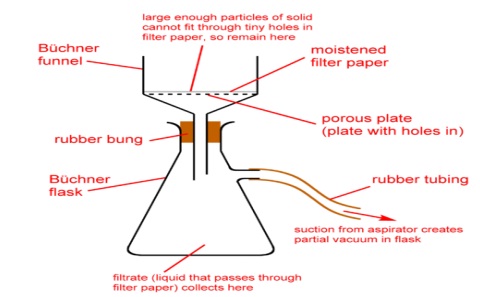
7. Filter the solid aspirin through a piece of pre-weighed filter paper using a Buchner funnel and the aspirator. Wash the crystals with 2-3 ml of chilled water. The liquid is mostly water and can be washed down the sink. Allow the air to be drawn through the solid and filter paper for 15 minutes. Be sure to record the filter paper weight in your notebook.
8. Place the filter paper with the product in a watch glass and put it in the oven at 100 oC for about 30 min. until dry.
9. Put the dry aspirin and the filter paper into a pre-weighed plastic bag and weigh again.
10. Measure the melting point range with the Meltemp Apparatus (your instructor will demonstrate) and compare to the value for pure aspirin of 138-140 oC.
11. Calculate the weight of your product by subtracting the weight of the paper and bag from the total. The theoretical (maximum) yield is 3.9 grams. What percent of this did you get? This is your percent yield. Record it in your notebook and turn in your product to the Stockroom.
Report the theoretical yield and the percent yield of the aspirin product.
Compare the melting point of you aspirin product to the theoretical melting point (138-140 C). Is the crude product above of below this mark?Explain why this is the case.

Determine the moles of aspirin from the titration and calculate the percent purity of the crude aspirin product from the titration analysis.
Plot a Beer’s Law graph of the standard 0.15% salicylic acid solution (C) on excel with Absorbance (yaxis) vs % salicylic acid (x-axis). To make a Beer’s Law plot, enter % Salicylic Acid into one column on the excel spread sheet (0 and 0.15) and absorbance into the next column (0 and the absorbance 5 reading).

Highlight the data and select insert, and scatter (w/ only markers). Right click a data point and select add trend line. Now set the y-intercept to 0 and check the display equation box. To add titles to the plot, select layout, axis titles, and then chart titles.
The % concentration for samples A and B can be determined by plugging in the absorbance readings for those samples into the linear equation and solving for x. Clearly state if the samples are below the maximum allowable limit for percent salicylic acid (0.15 %).
1. http://www.teachersfirst.com/lessons/forensics/ink-lab.html
2. www.reachoutmichigan.org/funexperiments/quick/csustan/mrsketch.htm