





Published on Apr 02, 2024
This project look at the technique called Sterilization of Water by using Bleaching Powder, which is used to purify water and make it fit for drinking.
Water is an important and essential ingredient in our quest for survival on this planet. It is very essential for carrying out various metabolic processes in our body and also to carry out Hemoglobin throughout the body. A daily average of 1 gallon per man is sufficient for drinking and cooking purposes. With the increasing world population, the demand for drinking water has also increased dramatically and therefore it is very essential to identify resources of water from which we can use water for drinking purposes. Since many available resources of water do not have it in drinkable form, in order to fulfill the demand of water, it needs to be purified and supplied in an orderly and systematic way.
There are many methods for the purification of water, such as:
1. Boiling
2. Filtration
3. Bleaching powder treatment
4. SODIS (Solar Water Disinfection)
Therefore we need a purification technique which can be used anytime and anywhere, does not require the use of any third party content and which is also economically feasible on both normal scale and large scale. Hence we look at the method of purification of water using the technique of treatment by bleaching powder commonly known as “Chlorination”.
In 1854 it was discovered that a cholera epidemic spread through water. The outbreak seemed less severe in areas where sand filters were installed. British scientist John Snow found that the direct cause of the outbreak was water pump contamination by sewage water. He applied chlorine to purify the water, and this paved the way for water disinfection. This discovery led to governments starting to install municipal water filters (sand filters and chlorination). So in the 1890s America started building large sand filters to protect public health. These turned out to be a success. Instead of slow sand filtration, rapid sand filtration was now applied. Subsequently, Dr. Fuller found that rapid sand filtration worked much better when it was preceded by coagulation and sedimentation techniques.
But the victory obtained by the invention of chlorination did not last long. After some time the negative effects of this element were discovered. Chlorine vaporizes much faster than water, and it was linked to the aggravation and cause of respiratory disease. Water experts started looking for alternative water disinfectants. In 1902 calcium hypo chlorite and ferric chloride were mixed in a drinking water supply in Belgium, resulting in both coagulation and disinfection. To this day, bleaching powder remains the most commonly used drinking water disinfectant. Almost all systems use some type of chlorine-based process to disinfect water. In addition to controlling disease-causing organisms, chlorination offers a number of benefits including:
• Reduces many disagreeable tastes and odors
• Eliminates slime bacteria, molds and algae that commonly grow in water supply reservoir
• Removes chemical compounds that have unpleasant tastes and hinder disinfection
• Helps remove iron and manganese from raw water.
For more than a century, the safety of drinking water supplies has been greatly improved by the addition of bleaching powder. However, bleaching powder also reacts with the organic matter, naturally present in water, such as decaying leaves thus forming a group of chemicals known as disinfection by-products. When used with modern water filtration methods, chlorine is effective against virtually all microorganisms. Bleaching powder is easy to apply and small amounts of the chemical remain in the water as it travels in the distribution system from the treatment plant to the consumer’s tap, thus ensuring prevention of recontamination of water.
Bleaching powder or Calcium hypochlorite is a chemical compound with formula Ca(ClO)2. This chemical is considered to be relatively stable and has greater available chlorine than sodium hypochlorite (liquid bleach). It is prepared by either calcium process or sodium process.
2Ca(OH)2 + 2 Cl2 --> Ca(ClO)2 + CaCl2 + 2 H2O
2Ca(OH)2 + 3Cl2 + 2NaOH ---> Ca(ClO)2 + CaCl2 + 2H2O + 2NaCl
What are the actual processes involved in disinfecting and purifying water?
The combination of following processes is used for municipal drinking water treatment worldwide:
1. Pre-chlorination - for algae or any biological growth control
2. Aeration - removal of dissolved iron and manganese
3. Coagulation - for flocculation
4. Coagulant aids also known as polyelectrolyte’s - to improve coagulation and for thicker floc formation
5. Sedimentation - for solids separation i.e. removal of suspended solids trapped in the floc
6. Filtration - for removal of carried over floc
7. Disinfection - for killing bacteria
Out of these processes, the role of Bleaching powder is only in the last step i.e. for Disinfection of water.
To determine the dosage of bleaching powder required for sterilization or disinfection of different samples of water.
Burette, titration flask, 100ml graduated cylinder, 250ml measuring flask, weight box, glazed tile, glass wool.
Bleaching Powder, Glass wool, 0.1 N Na2S2O3 solution, 10% KI solution, different samples of water, starch solution.
1. Bleaching powder when dissolved in contains dissolved chlorine, liberated by the action of bleaching powder with water.
Ca(OCl)2+H20 ---> Ca(OH)2+Cl2
2. The amount of Chlorine present is determined by treating a known volume with excess of 10% KI solution, when equivalent amount of I2 is liberated. The I2, thus liberated is then estimated by titrating it against a standard solution of Sodium thiosulphate, using starch solution as indicator.
Cl2+2KI ---> 2KCl+I2
2Na2S2O3 I2+ ---> Na2S4O6+2NaI
1. Preparation of bleaching powder solution Weigh accurately 2.5g bleaching powder and transfer it to a 250ml conical flask. Add about 100ml of distilled water. Stopper the flask and shake it vigorously. The suspension thus obtained is filtered through glass wool and the filtrate is diluted with water to make the volume 250ml. The solution obtained is 1% bleaching powder solution.
2. Take 20ml of bleaching powder solution in a stoppered conical flask and add it to 20ml of 10% KI solution. Stopper the flask and shake it vigorously. Titrate this solution against 0.1N Na2S2O3 solution taken in the burette. When the solution in the conical flask becomes light yellow in color, add about 2ml starch solution. The solution now becomes blue in color. Continue titrating till the blue color just disappears. Repeat the titration to get a set of three concordant readings.
Volume of bleaching powder sol. taken 20ml
Volume of KI solution added 20ml
Volume of different samples of water 100ml
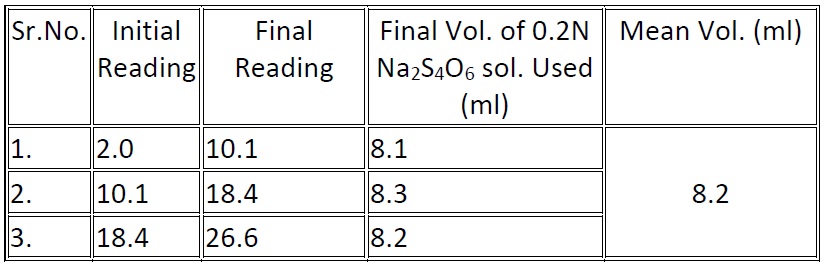
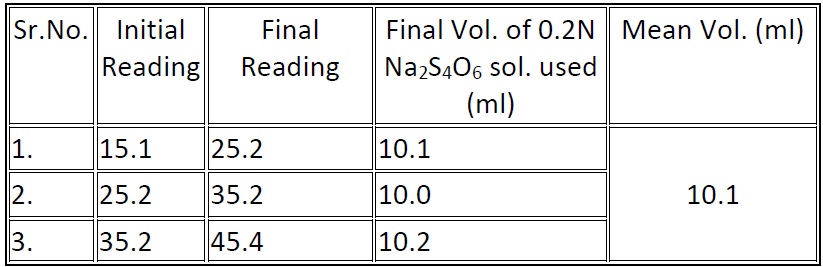
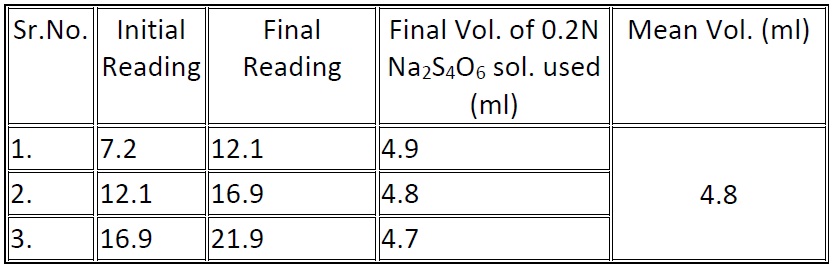
Amount of bleaching powder used to disinfect 100ml of tap water = (8.2 – 10.1) ml of 0.2 N of Na2S4O6 solution
= 1.9ml. Of 0.2 N of Na2S4O6 solution
Since, 250ml bleaching powder solution contains 2.5g bleaching powder
Thus, 1ml of bleaching powder solution contains bleaching powder =2.5/250 = 0.01g
Also, 20ml of bleaching powder solution = 8.2ml of 0.2N of Na2S2O3
So 1ml of Na2S2O3 solution = 20/8.2 ml of bleaching powder solution.
Volume of bleaching powder solution used to disinfect 100ml of water = 1.9x20/8.2ml.
1.9 x 20/8.2 ml. of bleaching powder solution =1.9x20x0.01/8.2 (gm) Bleaching Powder
Amount of bleaching powder used to disinfect 1 ltr. of water = 1.9x20x0.01x1000/8.2x100 = 0.4634gm
Amount of bleaching powder used to disinfect 100ml of water.
= (8.2 – 4.8) ml of 0.2 N Na2S2O3 solution
= 3.4ml
Accordingly,
Volume of Ca(OCl)2 solution required to disinfect 1lt. of water
= 3.4x20x0.01x1000/8.2x100
= 0.8293 gm.
Amount of the given samples of bleaching powder required to disinfect one liter of water:-
Samples I = 0.4634gm
Samples II = 0.8293 gm
Since amount of bleaching powder required for disinfecting POND WATER is more than that required for TANK WATER, thus it can be concluded that former contains more impurities.
While household bleaching solutions are widely available but it is not recommended to use it for household water treatment. If bleach is used for household water treatment system, concentration should be regularly checked and proper dosage strategy should be developed recommended by authorized organizations.
Bleaching Powder water treatment is useful in disinfecting water in places or conditions where boiling method cannot be practiced.
1. google.com
2. wikipedia.org
3. vlib.us
4. toppersarena.com
5. jmooneyham.com
6. ianrpubs.unl.edu