





Published on Apr 02, 2024
In this project, we shall investigate various factors such as nature of liquid, surface of liquid and temperature and find their correlation with the rate of evaporation of different liquids.
When a liquid is placed in an open vessel, it slowly escapes into gaseous phase eventually leaving the vessel empty. This phenomenon is known as vaporization or evaporation. Evaporation of liquids can be explained in the terms of kinetic molecular model although there are strong molecular attractive forces which hold molecules together. The molecules having sufficient kinetic energy can escape into gaseous phase. If such molecules happen to come near the surface in a sample of liquid all the molecules do not have same kinetic energy.
There is a small fraction of molecules which have enough kinetic energy to overcome the attractive forces and escapes into gaseous phase. 3 4 Chemistry project Evaporation causes cooling. This is due to the reason that the molecules which undergo evaporation have high Kinetic energy therefore the kinetic energy of the molecules which are left behind is less. Since the remaining molecules which are left have lower average kinetic energy. Therefore temperature is kept constant the remaining liquid will have same distribution of the molecular kinetic energy and high molecular energy will kept one escaping from liquid into gaseous phase of the liquid is taken in an open vessel evaporation will continue until whole of the liquid evaporates.
Evaporation is an essential part of the water cycle. Solar energy drives evaporation of water from oceans, lakes, moisture in the soil, and other sources of water. In hydrology, evaporation and transpiration (which involves evaporation within plant stomata) are collectively termed evapotranspiration. Evaporation is caused when water is exposed to air and the liquid molecules turn into water vapour which rises up and forms clouds.
If the air already has a high concentration of the substance evaporating, then the given substance will evaporate more slowly.
If the air is already saturated with other substances, it can have a lower capacity forth substance evaporating.
If the substance is hotter, then evaporation will be faster.
This is in part related to the concentration points above. If fresh air is moving over the substance all the time, then the concentration of the substance in the air is less likely to go up with time, thus encouraging faster evaporation. In addition, molecules in motion have more energy than those at rest, and so the stronger the flow of air, the greater the evaporating power of the air molecules.
The stronger the forces keeping the molecules together in the liquid or solid state the more energy that must be input in order to evaporate them.
The rate of evaporation of liquids varies directly with temperature. With the increase in the temperature, fraction of molecules having sufficient kinetic energy to escape out from the surface also increases. Thus with the increase in temperature rate of evaporation also increases. Molecules that escape the surface of the liquids constitute the evaporation. Therefore larger surface area contributes accelerating evaporation.
The magnitude of inter-molecular forces of attraction in liquid determines the speed of evaporation. Weaker the inter-molecular forces of attraction larger are the extent of evaporation. In diethyl ether rate of evaporation is greater than that of ethyl alcohol.
The rate of evaporation of liquids depends upon the flow of air currents above the surface of the liquid. Air current flowing over the surface of the liquid took away the molecules of the substance in vapour state thereby preventing condensation.
The higher the density, the slower a liquid evaporates. In the US, the National Weather Service measures the actual rate of evaporation from a standardized "pan" open water surface outdoors, at various locations nationwide. Others do likewise around the world. The US data is collected and compiled into an annual evaporation map. The measurements range from under 30 to over the120 inches (3,000 mm) per year.
In an area of less pressure, evaporation happens faster because there is less exertion on the surface keeping the molecules from launching themselves
When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. This is accelerated by factors such as low humidity, heat (from the sun), and wind. In a cloth dryer hot air is blown through the clothes, allowing water to evaporate very rapidly.
For molecules of a liquid to evaporate, they must be located near the surface, be moving in the proper direction, and have sufficient kinetic energy to overcome liquid -phase intermolecular forces. Only a small proportion of the molecules meet these criteria, so the rate of evaporation is limited. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. As the faster-moving molecules escape, the remaining molecules have lower average kinetic energy, and the temperature of the liquid thus decreases. This phenomenon is also called evaporative cooling. This is why evaporating sweat cools the human body. Evaporation also tends to proceed more quickly with higher flow rates between the gaseous and liquid phase and in liquids with higher vapour pressure. For example, laundry on a clothes line will dry (by evaporation) more rapidly on a windy day than on a still day. Three key parts to evaporation are heat, humidity and air movement.
To compare the rates of evaporation of acetone, benzene and chloroform.
Three same size Petri dishes of diameter 10 cm, 10 ml. pipettes, stop watch, acetone benzene and chloroform.
1. Clean and dry all Petri dishes and identify them as A, B and C.
2. Pipette out of 10 ml. acetone in Petri dish "A" with stopper similarly pipette out 10 ml of benzene and chloroform in each of Petri "B" and "C".
3. Remove the cover plates from all Petri dishes and start the stop watch.
4. Let the Petri dishes remain exposed for 10 minute. Now cover each of the Petri dish and note the volume of remaining material in them.
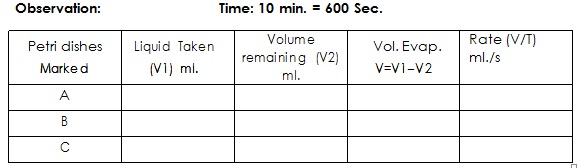
1. Rate of evaporation of Acetone is ml/s.
2. Rate of evaporation of Benzene is ml/s.
3. Rate of evaporation of Chloroform is ml/s.
The intermolecular forces of acetone, benzene and chloroform are in order.
Chloroform > Benzene > Acetone.
To study the effect of surface area on the rate of evaporation of diethyl ether.
Three Petri dishes of diameter 2.5 cm, 5 cm, 7.5 cm. with cover 10 ml. of pipette and stop watch.
1. Clean and dry all Petri dishes and mark them as A, B and C.
2. Pipette out of 10 ml. diethyl ether in each of the Petri dishes A, B and C and cover them immediately.
3. Uncover all three Petri dishes and start the stop watch.
4. Note the remaining volume after 10 min. vaporization of diethyl ether from each Petri dish.

The order of evaporation of acetone in three Petri dishes as
Larger the surface area more is evaporation.
To study the effect of temperature on the rate of evaporation of acetone.
Two Petri dishes of 5 cm. diameter each stop watch, 10 ml. pipette, thermometer and thermostat.
1. Wash and Clean, dry the Petri dishes and mark them as A, B.
2. Pipette out of 10 ml. of acetone to each of Petri dishes A and B and cover them.
3. Put one Petri dish at room temperature and to the other heat for same time.
4. Note the reading.
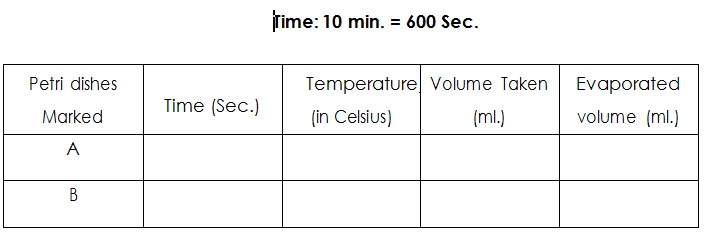
The order of evaporation of acetone in two Petri dishes as given
Room Temperature ----- Heating
Observation clearly shows that the evaporation increases with temperature.
To study the effect of air current on the rate of evaporation of acetone.
Two Petri dishes acetone.
1. Clean and dry the Petri dishes and mark them as A and B.
2. Keep one dish where no air current and other under a fast air current.
3. Note the reading.
• Initial Volume 10 ml. of Acetone.

The order of evaporation of acetone in two Petri dishes as given
With fan ------ Without Fan.
The rate of evaporation of liquid increases with the increase in rate of flow of air current.
1. www.google.com
2. www.wikipedia.org
3. www.allprojectreports.com
4. www.chemistryprojects.com
5. Comprehensive practical book