





Published on Apr 02, 2024
To determine the rate of fermentation of various fruit juices.
Fermentation is a slow decomposition of complex organic compound into simpler compounds by the action of enzymes. Enzymes are generally protons example of fermentation are souring of milk curd, bread making wine making, rewing.
The word ‘Fermentation’ had been derived from latin (Ferver means to ‘boil’). As during fermentation there is lot of frothing of liquid due to the evolution of carbon dioxide, it gives the appearance as if it is boiling. Sugars like glucose, sucrose when fermented in presence of yeast cells are converted to ethyl alcohol. During fermentation of, starch is first hydrolyzed to maltose by action of enzyme diastase.
Fermentation is carried out at a temperature of 4-16°C (40-60°F). This is slow for most kinds of fermentation, but is beneficial for cider as it leads to slower fermentation with less loss of delicate are ma. Apple based juices with cranberry also make fine ciders and many other fruit purees or flavorings can be used, such as grapes, cherry, raspherry. The cider is ready to drink after a three month fermentation period, though more after it is material in the vats for up to 2 to 3 years
Since fruits ferment naturally, fermentation precedes human history. Since ancient times, however, humans have been controlling the fermentation process. The earliest evidence of winemaking dates from eight thousand Years ago in Georgia, in the Caucasus area. Seven thousand years ago jars containing the remains of wine have been excavated in the Zagros Mountains in Iran, which are now on display at the University of Pennsylvania.There is strong evidence that people were fermenting beverages in Babylon circa 5000 BC, ancient Egypt circa 3150 BC, pre-Hispanic Mexico circa 2000 BC,and Sudan circa 1500 BC.There is also evidence of leavened bread in ancient Egypt circa1500 BC and of milk fermentation in Babylon circa 3000 BC.French chemist Louis Pasteur was the first known zymologist, when in 1854 he connected yeast to fermentation. Pasteur originally defined fermentation as “respiration without air”.
Louis Pastuer in 1860 demonstrated that fermentation is a purely physiological process carried out by living microorganism like yeast. This view was abandoned in 1897 when Buchner demonstrated that yeast extract could bring about alcoholic fermentation in the absence of any yeast cell. He proposed that fermenting activity of yeast is due to active catalysts of biochemical origin. These biochemicals are called enzymes. Enzymes are highly specific compound or a closely related group of compounds.
Fermentation has been utilized for many years in the preparation of beverages. Materials from Egyption tombs demonstrated the procedures used in making beer and leavened bread. The history of fermentation, whereby sugar is converted to ethanol by action of yeast, is also a history of chemistry. Van Helmont coined the word iogaslt in 1610 to describe the bubble produced in fermentation.
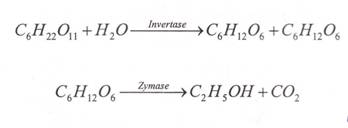
Leeuwenhoek observed anddescribed the cell of yeast with his newly invented microscope in 1680. The fruit and vegetable juices contain sugar such as sucrose, glucose, and fructose. These sugars of fermentation in presence of enzyme invert are and zymose give with the evolution of 〖CO〗_2. Maltose is converted to glucose by enzyme maltose, glucose to ethanol by another enzyme zymose.
Enrichment of the diet through development of a diversity of flavors, aromoas and textures in food substances.
Preservation of substantial amounts of food through lactic acid alcohol, acetic acids and alkaline fermentation.
Biological enrichment of food substances with proteins, essential aminoacids, falty acids and vitamins.
Elimination of anti nutrients.
A decrease in cooking times and fuel requirements.
Fruit juices contain various sugars like glucose, fructose etc. When juices are treated with yeast and it converts sugar into glucose and fructose. These monosccharides are further converted into ethyl alcohol by another enzyme known as zymose.
The relative rates of fermentation can be established with fillings solution A and B. Since glucose in an aldose gives red precipitate with fellings solution. When all the quantity of glucose is converted to ethanol, the mixture will not give red
Fruits such as pineapple, apple, orange, grape, lemon, yeast powder, ammonium sulphate , fehling's solutions, beaker roundbottom flask, thermometer,test tubes, dropper, stand, hot water both, conical flask, distilled water etc.
Step 1 : Take approximately Ig of yeast powder in beaker, add 20ml distilled water and 3 -4ml of saturated solution of ammonium sulphate^tir the solution by a glass rod.
Step 2: Pour 2ml of fruit juice a clean round bottom flask and add 20ml of distilled water.
Step 3: Now transfer the content of the beaker into a round bottom flask and shake the mixture.
Step 4: Place the round bottomed flask into a hot water bath containing water at 35 - 45°C and shake the solution after each minute.
Step 5: After keeping the round bottomed flask for 10 min, take out 10 drops of the mixture in a test tube and add 1 ml of "Fehling's solution -B". Heat the test
tube in a hot water both for few minutes observe. The change in color and the fermentation of red precipitate. Perform this test after internal of five minutes until the mixture gives red precipitate with Fehling's reagent.
Step 6: Repeat the procedure in the same way taking other samples of fruit juices.
All fruit juices do not undergo fermentation at the same rate. The increasing order of the rate of fermentation is:
Apple <Pine apple juice = orange juice <Grape juice < Lemon Juice.
• PRACTICAL CHEMISTRY COMPREHENSIVE - XII
• NCERT TEXT BOOK-XII
• INTERNET