https //phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom en.html
Build an atom from scratch, using protons, neutrons, and electrons. Test different combinations to produce ions and unstable elements.
PhET sims are fun, interactive, research-based simulations of physical phenomena developed by the PhET™ project at the University of Colorado. Conversion to HTML5 and tablet compatibility supported by the Royal Society of Chemistry.
Sample Learning Goals:
- Use the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass and charge.
- Predict how addition or subtraction of a proton, neutron, or electron will change the element, the charge, and the mass.
- Use the element name, mass, and charge to determine the number of protons, neutrons, and electrons.
- Define proton, neutron, electron, atom, and ion.
- Generate an isotopic symbol for an atom, given the number of protons, neutrons, and electrons
aainflight.com  Login to Watch Movies: What’s On My AA Flight?
Model Simplifications
• Although the title of the sim is “Build an Atom”, students can build both neutral atoms and ions.
• The nucleus is magnified to allow students to see the number of protons and neutrons.
• The radii of the orbits in the Bohr model are not in the correct ratio.
• In the “Cloud” model, the shape of the cloud is not meant to represent orbitals and the size of the cloud does not represent actual atomic or ionic radii. The cloud simply gets larger and darker as the number of electrons in the cloud increases.
• We define “Stable” as an isotope whose half-life is too long to be measured. The nucleus of an “Unstable” atom vibrates but does not fall apart.
• Students can create ions that are not found in nature (for example, He+2). Students can still reach suggested learning goals related to net charge on ions even if not all ions they create exist in nature.
• Excited states are not allowed in the sim. In the Bohr model, if a core electron is removed, an outer electron will move to the inner shell. The sim does not show the subsequent release of a photon due to this electron movement.
• The Symbol representation uses the standard isotope notation with the mass number on the top and the atomic number on the bottom. This may differ from how the data is displayed in some periodic tables (atomic number at the top). Students who are unfamiliar with the isotope notation may require additional scaffolding.
Bankmobile Vibe Activate Card : How do I Activate my BankMobile card?
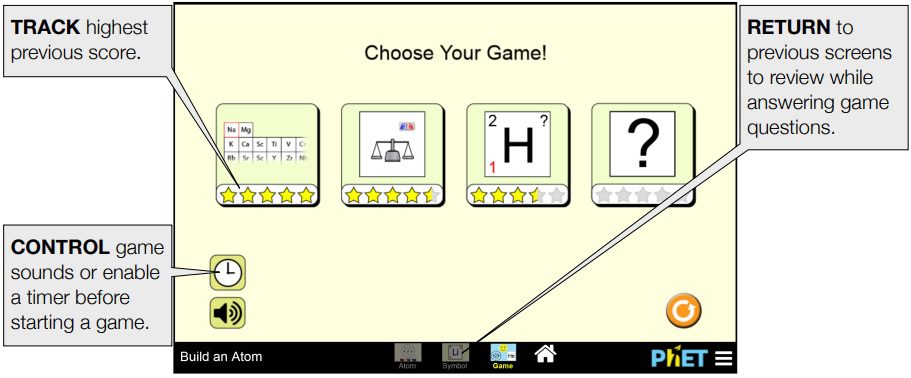
Game Screen
Students are presented with 5 challenge questions in each game.
Game 1 – Identify the element when provided a model or count of subatomic particles.
Game 2 – Calculate the mass number or charge of an atom or ion.
Game 3 – Interpret atomic symbols
Game 4 – Mixed review
Be the first to comment