





Published on Jan 09, 2026
Liquid Nitrogen is the cheapest, widely produced and most common cryogenic liquid. It is mass produced in air liquefaction plants. The liquefaction process is very simple in it normal, atmospheric air is passed through a dust precipitator and pre-cooled using conventional refrigeration techniques.
It is then compressed inside large turbo pumps to about 100 atmospheres. Once the air has reached 100 atmospheres and has been cooled to room temperature it is allowed to expand rapidly through a nozzle into an insulted chamber. By running several cycles the temperate of the chamber reaches low enough temperatures the air entering it starts to liquefy. Liquid nitrogen is removed form the chamber by fractional distillation and is stored inside well-insulated Dewar flasks.
Heat from the atmosphere vaporizes liquid nitrogen under pressure and produces compressed nitrogen gas. This compressed gas runs a pneumatic (compressed gas drive) motor with nitrogen gas as the exhaust.
Main Components of the Engine:
• A pressurized tank to store liquid nitrogen
• A heat exchager that heats (using atmospheric heat) liquid nitrogen to form nitrogen gas, then heats gas under pressure to near atmospheric temperature.
• A pneumatic motor (along with a Volkswagen transmission) that runs the car.
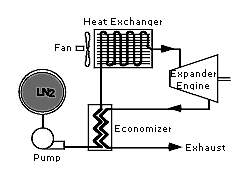
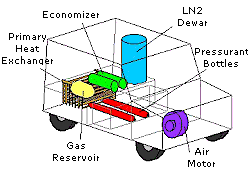
The principle of running the LN2000Car is like that of steam engine, except there is no combustion involved. Instead liquid nitrogen at -320 o F (-196 o C) is pressurized and then vaporized in a heat exchanger by ambient temperature of the surroundings air. This heat exchanger is like the radiator of a car but instead of using air to cool water, it uses air to heat and boil liquid nitrogen. The resulting high pressure nitrogen gas is fed to an engine that operates like a reciprocating steam engine, converting pressure to mechanical power. The only exhaust is nitrogen, which is major constituent of our atmosphere.
A single-cylinder reciprocating expander that runs on compressed nitrogen gas with the exhaust gas released into the atmosphere was considered. When compressed gas flowed into the expanders cylinder, isobaric work was done on the moving piston by the gas. The net isobaric expansion work done during a single cycle is gauge pressure of the gas multiplied by the volume of the gas that flows into the cylinder.
The isobaric specific energy is Wi = (Ph-Pi)V= Ph(1-P-1)V
Ph-Pi is the difference in absolute pressure between inlet and exhaust gas.
If Pi is atmospheric pressure, Ph-Pi is the gauge pressure of compressed gas.
V is the volume occupied by the compressed gas per unit mass of gas.
P = Ph / Pi is inlet to exhaust pressure ratio.
The isobaric specific energy is Wi = RTh (1-P-1) /A.
Here Th refers to the temperature of the high pressure inlet gas
The COOLN2Car which a converted 1973 Volkswagen and runs on liquid nitrogen is an illustrative to the use of isobaric expansion equation.
The processes considered are the expansion of nitrogen gas at 300K and 3.3 MPA to near atmospheric pressure. The first process considered is isothermal expansion from 3.3 MPA to 120KPA and the work can be easily computed as
Wisothermal = rT ln (P2/P1)
r = 0.2968 (KJ/KgK) for nitrogen gas and T = 300K.
The result for Nitrogen is 291.59 KJ/Kg. Another limiting process is the simple adiabatic expansion of the gas in which no heat is admitted during. the expansion. The work is calculated as
Wadiabatic = KrT [1-(P2 / P1) K-1/K] (k-1)
Where T = 300K and K = 1.4, the ratio of specific heats for nitrogen.
The resulting Wadiabatic is 180KJ/Kg of Nitrogen exhausted at 150KPA.
The energy density of liquid nitrogen is relatively low and better than readily available battery systems.
They have significant performance and environmental advantages over electric vehicles.
A liquid nitrogen car is much lighter and refilling its tank will only 10-15 minutes.
The exhaust produced by the car is environmental friendly.
The cool LN2 car can travel 15 miles on a full (48 gallon) tank of liquid nitrogen going 20 MPH. Its maximum speed is over 35 MPH.
In a real sense, the more such vehicles are used, the cleaner the air will become if the liquefaction process is driven by non-polluting energy sources. In addition to the environmental impact of these vehicles, refueling using current technology can take only a few minutes, which is very similar to current gas refueling times.
Research paper on “Liquid Nitrogen as a Non-Polluting Vehicle Fuel” by Mitty c. Plummer, Carlos A. Ordonez and Richard F. Reidy, niversity of North Texas.
The University of Washington’s Liquid Nitrogen Propelled Automobile
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |