





Published on Jan 09, 2026
Iontophoresis is an effective and painless method of delivering medication to a localized tissue area by applying electrical current to a solution of the medication. The delivered dose depends on the current flowing and its duration.
Iontophoresis is a recognized therapeutic method for delivering ionic compounds, i.e. drugs, into and through the skin by applying electrical current. It has proven to be a beneficial treatment for many localized skin disorders such as; nail diseases, Herpies lesions, psoriasis, eczematous, and cutaneous T-cell lymphoma. The method has also been reported useful for topical anesthesia to the skin prior to cut-down for artificial kidney dialysis, insertion of tracheotomy tubes and infiltration of lidocaine into the skin prior to venipuncture.
Treatment of various musculoskeletal disorders with anti-inflammatory agents has been reported in the literature. Iontophoresis enhances the transdermal delivery of ionized drugs through the skin's outermost layer (stratum corneum) which is the main barrier to drug transport. The absorption rate of the drug is increased, however, once the drug passes through the skin barrier natural diffusion and circulation are required to shuttle the drug to its proper location.
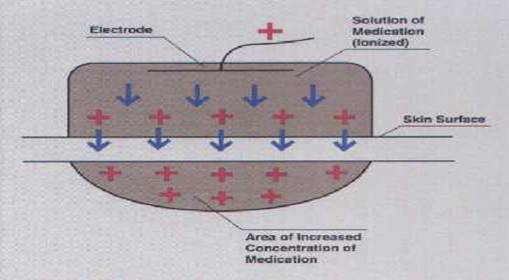
The mechanism by which iontophoresis works is based upon the knowledge that like electrical charges repel. Application of a positive current from an electrode to a solution applied to a skin surface will drive the positively charged drug ions away from the electrode and into the skin. Obviously, negatively charged ions will behave in the same manner.
By definition, iontophoresis is the increased movement of ions in an applied electric field. Iontophoresis is based on the general principle that like charges repel each other and unlike charges attract each other.
An external energy source can be used to increase the rate of penetration of drugs through the membrane. When a negatively charged drug is to be delivered across an epithelial barrier which is placed under the negatively charged delivery electrode (cathode) from which it is repelled, to be attracted to the positive electrode placed elsewhere on the body. In anodal iontophoresis (positively charged ions), the electrode orientation is reversed (fig.1).
The choice of drug is of importance depending on whether the compound is unionised or ionised. Non-ionised compounds are generally better absorbed through the skin than ionised substances. The penetration across the skin or other epithelial surfaces is usually slow due to their excellent barrier properties. Many drug candidates for local applications only exist in an ionised form, which makes effective membrane impossible.
Many factors have been shown to affect the results of iontophoresis. These include the physiochemical properties of the compound (molecular size, charge, concentration), drug formulation, type of vehicle, buffer, pH, viscosity, pressure of other ions), equipment used (available current range, constant versus pulsed current, type of electrode), biological variations (skin site, regional blood flow, age, sex), skin temperature and duration of iontophoresis.
Iontophoresis has mainly been used for therapeutic purposes, but in combination with the laser Doppler technique, it is possible to study the influence of the drugs used on vascular bed. Until now, the combination LDPM (laser Doppler perfusion monitoring) and iontophoresis has been used mostly as a diagnostic tool for diseases affecting macro- and microcirculation and the controlling regulatory nerves. When using iontophoresis as a diagnostic instrument, the following factors have to be considered.
The pH is of importance for the iontophoretic delivery of drugs .The optimum is a compound that exists predominantly in an ionised form. When the pH decreases, the concentration of hydrogen ions increases, and a vascular reaction (vasodilatation) is initiated because of C-fibre activation (fig.2). Thus, it is important to keep the pH as close as possible to 7, atleast when working with vasodilators. At pH 5.5 and below, there is an increasing risk for vascular reactions due to the high concentration of hydrogen ions rather than the compound used. Since hydronium ions are small, they penetrate the skin more easily than larger drug ions.
There is a linear relationship between the observed flux of a number of compounds and the applied current. With the present electrode area of 1 cm2, the current is limited to 1mA due to patient comfort considerations. This current should not be applied for more than 3 minutes because of local irritation and burns. With increasing current, the risk of non-specific vascular reactions (vasodilatations) increases. At a current of 0.4 to 0.5 mA/cm2, such a vascular reaction is initiated after a few seconds of iontophoresis with deionised or tap water. This latter effect is probably due to the current density being high enough within a small area to stimulate the sensory nerve endings, causing reactions such as the release of substance P from C-fibre terminals. (Fig.2)
In a solution of sodium chloride, there is an equal quantity of negative (Cl-) and positive (Na+) ions. Migration of a sodium ion requires that an ion of the opposite charge be in close vicinity. The latter ion is of opposite charge is referred to as a counter-ion. An ion of equal charge but of a different type is referred to as a co-ion.
When using iontophoresis, it is important to know that adding buffering agents performs pH adjustment. The use of buffering agents adds co-ions, which are usually smaller and more mobile than the ion to be delivered. This results in a reduction of the number of drug ions to be delivered through the tissue barrier by the applied current. In our example, this means that when a positively charged drug is diluted in saline, the sodium ions will compete with the amount of drug ions to be delivered. Ideally, the use of a buffer system should be avoided in iontophoresis, but if this is not possible, alternative buffers consisting of ions with low mobility or conductivity are preferred.
Depending on the drug used, the steady-state flux (ion movement) has been shown to increase with increasing concentration of the solute in the donor compartment that is in the delivery electrode. A limiting factor to be considered is the strength of the current used. At higher drug concentrations, the transport may be independent of concentration probably because of the saturation of the boundary layer relative to the bulk solution.
It has been shown that the permeability coefficients in positively charged, negatively charged and uncharged solutes across excised human skin are a function of molecular size. When the molecular size increases, the permeability coefficient decreases. However, there are certain solutes with relatively high molecular size (e.g. insulin, vasopressin and several growth hormones) that have also been shown to penetrate the skin barrier into the systemic circulation.
When performing iontophoresis with a specific current the flow of ions across the membrane induces a flow of solvent called electro-osmosis. Compared to the ion transport the electro-osmotic contribution is small. The penetration of the uncharged substances (e.g. bovine serum albumin) has been shown to be facilitated by the volume flow effect induced by an applied potential difference across the membrane. Iontophoresis has also been observed to enhance the penetration of a number of dipolar ions (zwitterionic substances, such as phenylalanine). Most of the substances have been shown to be delivered in significantly higher amounts in anodic delivery than by cathodic delivery. In general, iontophoresis is more effective for charged compounds, especially monovalent ions.
Application of a continuous current over a long period of time can modulate iontophoretic delivery. Continuous DC current may result in skin polarisation, which can reduce the efficiency of iontophoretic delivery in proportion to the length of current application. This polarisation can be overcome by using pulsed DC, a direct current that is delivered periodically. During the “off-time” the skin becomes depolarised and results to its initial unpolarised status. The enhanced skin depolarisation using pulsed DC can however decrease the efficiency of pulsed transport if the frequency is too high. Enhanced iontophoretic transport has been reported for peptides and proteins using pulsed DC compared to conventional DC.Most of the drug ions used for diagnostic purposes in combination with iontophoresis and LDPM are small in size. As a result, the time needed for an effect is relatively short (5-120sec), compared to when iontophoresis is used for therapeutic purposes (20-40min).
Iontophoresis reduces intra- and inter- subject variability in deliver rate. This is an inherent disadvantage with the passive absorption technique. Experiments in vivo and invitro gives support for clinical findings that there are small differences in the flux rate following transdermal iontophoresis between males and females, as well as between hairy and hairless skin. The status of the vascular bed is also important; for instance, a pre-constricted vascular bed decreases the drug flux through the skin while a dilated vascular bed increases the yield of drug through the skin.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |