





Published on Nov 30, 2023
Arsenic is one of the most toxic elements present in ground water and surface water, that threat human and other animals’ health. Arsenic (III) removal has been done with different kinds of methods. In this test an effort has been made to investigate the arsenic removal from ground water with iron nano particles (INP) to meet drinking water standard limit of 0.01 mg/L. In this experiment the INP prepared in the laboratory, were mixed with sample containing moderately amount of arsenic in them. The batch adsorption experiments were carried out with varying dosages of INP and pH alternatively at a constant period of 15 minutes. The test confirmed that 97.6% of arsenic was being removed from the sample at an optimum pH of 4 and an optimum dosage of 2 g/L. On the other hand analysis of synthetic sample was done to know the effect of initial Arsenic concentration in the sample.It was found that the efficiency of the process decreased as the initial arsenic concentration increased.
Keywords : INP- Iron Nano Particles, nzVI-Zero valent Iron Nano Particles
1. To analyse the water sample collected from Vandali village of Raichur District for Arsenic content
2. To synthesize Zerovalent Iron Nanoparticles(INP) in the Laboratory
3. To treat the Arsenic affected water sample with prepared INP at varying dosages of INP and pH
4. To know the efficiency of prepared INP at various dosages and pH in Arsenic Removal
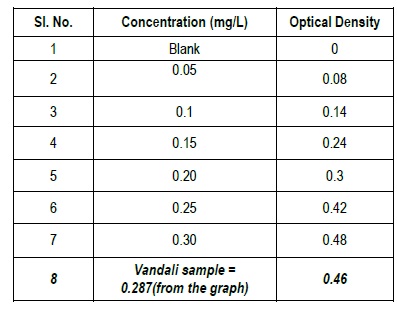
Gives the Optical densities for various concentrations of the standard prepared
A) Effect of pH on Efficiency:
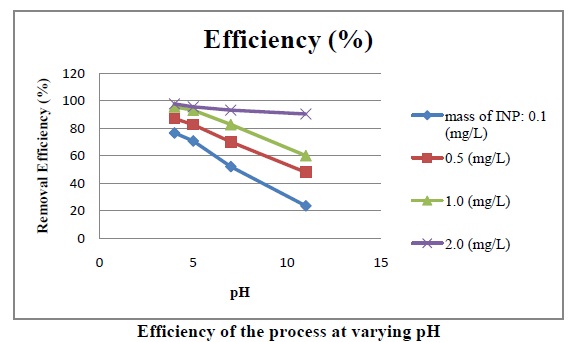
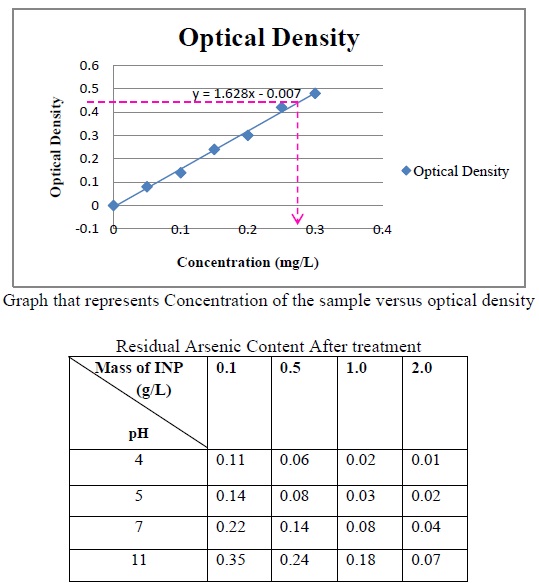
The above fig. shows that As (III) removal efficiency increases significantly with decreasing pH. The picture depicts the effect pH on the removal efficiency at varying mass of INP.
The pH dependent behaviour is explained by Ionization of both the adsorbate and adsorbent causing repulsion at the surface at the basic pH and decreasing the net As (III) adsorption
Under acidic condition the corrosion of Fe0 ions accelerates. Hence as the Fe0 ions corrodes and dissociates for adsorption of As
In basic condition when the ferrous ions dissolves from iron surface, collides with hydroxyl ions, it produces ferrous hydroxide precipitate that deposits on the reactive sites which hinder the reaction
It can be concluded that acidic condition is better for the treatment
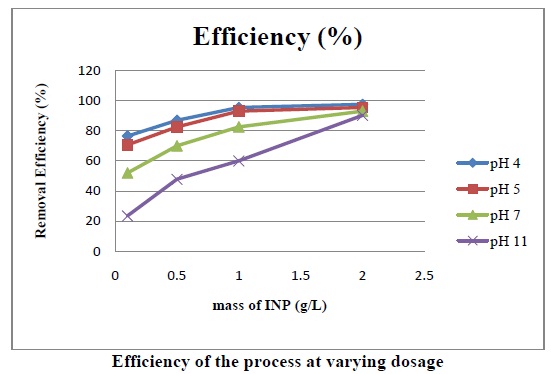
The above fig. shows that As (III) removal efficiency increases significantly with increase of Fe0 concentration. The picture depicts the effect of mass of INP added on the removal efficiency at varying pH.
Increased concentrations of INP provide more iron surface active sites for collision with As (III) molecules which accelerates the As (III) removal efficiencies and also increases oxidation and deduction reactions.
The higher As (III) removal with time elapse is due to the superior surface area of INP demanding a much lower dose than that of micro scale iron.
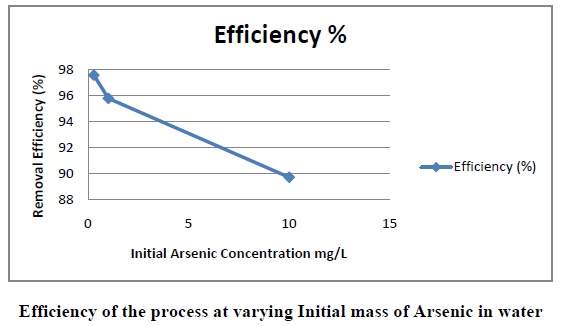
The above figure shows the efficiency of the process with increasing Initial concentration of Arsenic. The above analysis was done to efficiency of the process with increasing concentration of Arsenic. The results can be tabulated as follows:
As the concentration of Arsenic in the water increases the removal efficiency decreases of INP/process
• Few places of Raichur district which have contaminated groundwater, application of low cost and high performance method can be highly effective.
• Without any advanced facilities INP in very low scale could remove arsenic from aqueous solution at very short time over a wide range of pH.
• In processes, INP, efficiently increases by decreasing pH and increase in mass of INP added, efficiency of the process decreases with the increase in Initial Arsenic Concentration