





Published on Apr 02, 2024
This experiment is designed to illustrate the purpose of the different components of a photographic developer. It will also illustrate the chemistry of the toning process. Specifically, you will determine the role of one component in the developer and identify the importance of reaction duration as a variable in the toning of black and white prints. For the first part, be specific. Indicate which component you are omitting. Discuss the role of that component in your conclusion section.
Traditional black and white photography utilized a chemical process that has been observed since ancient times – that of the photo-sensitivity of silver (Ag) compounds. This ability was harnessed by the19th century photography pioneers by coating silver halides (molecules of Ag and halide gases) in an emulsion of gelatin onto a glass or plastic backer. The process of making traditional film has not changed much from the basic chemical reactions discovered in the 1800’s. Initially, elemental silver is reacted with nitric acid to form silver nitrate. Complete and balance the reaction below:
Ag (s) + HNO3 → AgNO3 + H2
Identify the element oxidized and that reduced in the above equation.
The silver nitrate is then converted to the silver halide (usually bromide) by fuming the film with potassium bromide. Complete and balance the next reaction:
AgNO3 + KBr →
The AgBr is an ionic compound that forms a crystalline structure in grains. What does it mean to be an ionic compound? The film now is ready for exposure and the creating of the intended image. Stored within a light sealed container, the AgBr film is a uniform darkish yellow. The light exposure occurs when an opening allows light to enter the dark container and strike the film. These “storage” containers that subsequently allow light exposure are more commonly referred to as cameras.
In the first section of this experiment you will determine a set of optimum conditions for developing a contact print using a light bulb for exposure and a standard, already-prepared developer. Two factors are important in determining the optimum conditions. The first is the amount of light hitting the photographic paper. This depends on the intensity of the light, the length of the exposure and the distance of the light from the paper. The greater the amount of light hitting the paper, the darker the print since more silver halide grains are exposed. This assumes all other conditions are held constant. The directions for the first part are listed in Section 1.
In the second section of the experiment you will prepare a developer by weighing out and mixing the necessary chemicals. You will then use the conditions determined in the first part to prepare a contact print with your own developer. Most photographic developers have several components in common. These are:
1. a reducing agent--to reduce the exposed silver halide grains to metallic silver,
2. a preservative--to prevent reaction of the reducing agent with oxygen from the air,
3. an activator--to activate the reducing agent,
4. a restrainer--to increase the contrast between light and dark areas in the print.
In your developer the chemicals will be:
1. metol, the reducing agent.
2. sodium sulfite (Na2SO3), the preservative.
3. sodium carbonate (Na2CO3•H2O), the activator.
4. sodium bromide (NaBr), the restrainer.
1. Obtain several sheets of photographic paper. Caution: this paper is, of course, very sensitive to light.
2. Place approximately 100 ml each of developer, stop bath, fixer, and distilled water into 400 ml beakers; label each beaker.
3. Set up the exposing light as shown in the Figure below.
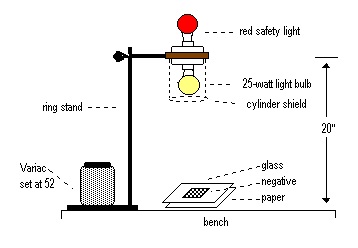
4. Using only safety lights, remove a piece of photographic paper from its storage space and place it directly under the light with the emulsion side up. Place the negative on top of the paper and put a glass slide on top of the negative.
5. Turn on the light for 30 seconds to expose the paper. Using only the safety light, observe the paper. Does it appear to have changed?
6. Develop the print (still under safety light) by placing it in the beaker containing the developer for 60 seconds (caution: use tongs), then the stop bath for 60 seconds, the fixer for 5 minutes and the distilled water for 5 minutes. While the paper is in the developer and stop bath, be certain that you agitate it. Place the print on a paper towel to dry. Record the temperature of the developer.
7. Try to improve the print. If it is too dark, reduce the exposure or the developing time. The exposure can be reduced by reducing the exposure time, raising the bulb, or reducing the setting on the Variac. If the print is too light, the opposite remedies should be applied. Be certain to keep an accurate record of your conditions for each experiment. A Table such as shown below works well. Your goal here is to find the conditions for the best print possible. You will use these conditions in the subsequent section for your developer. Put 1/2 of each print next to the conditions in youur Results section.

Prepare your own developer by dissolving 2.0 g Na2SO3, 0.25 g sodium bromide, NaBr, 0.6 g metol, and 2.0 g of sodium carbonate (Na2CO3·H2O) in distilled water and dilute to 100 ml. Be sure to dissolve these chemicals in the order listed.
Expose and develop a contact print again according to your optimum conditions of Section 1. Record your results. Try to improve the print.
How does this print compare with that of Section 1?
Prepare 8 extra prints for the toning experiments. Make these prints as soon as possible after your determination of the optimal conditions.
What happens if...? What happens if you prepare a developer but leave out one of the ingredients? Try it and record your results.
Prepare an iron toning bath by mixing 10.0 ml of ferric ammonium citrate (10% solution), 10.0 ml of K3Fe(CN)6 (10% solution) and 100 ml of a 10% solution of acetic acid in a 400-ml beaker. This solution can be safely disposed of in the sink.
Place a print in the iron toning solution for 5 minutes. What happens? How does the length of time in the toning solution affect the print? Test this by placing a print in the toning bath for only 2 minutes. Try another time for the toning bath. Rinse the print in deionized water briefly, and record your results.
Prepare a copper toning bath as follows. Dissolve 0.54 g of K3Fe(CN)6 and 2.65 g of potassium citrate in 100 ml of H2O. In a separate beaker, dissolve 0.66 g of copper sulfate and 2.65 g of potassium citrate in 100 ml of water. Mix equal volumes of the two solutions just prior to use. (Copper is a heavy metal. Dispose of this solution in the aqueous waste container).
Place a print in your bath for 5 minutes and rinse What do you see? How does the length of time in the bath affect the result?
Place a print in a 400 ml beaker containing about 100 ml of 20% sodium thiosulfate solution for 5 minutes. Without rinsing, immerse the print in a beaker containing the prepared hydrochloric acid for 30 minutes then rinse in distilled water. (Both of these solutions can be put down the drain with the water running). What do you observe? How does length of time in the acid bath affect the result? (Note: In your Results section, include the prints by taping them in the appropriate section).
For your conclusion describe the role of the chemical which you left out of the developer. Refer to the mechanism of the action of the black and white developer as described by your lab instructor and in the handout. Also draw a conclusion about the effect of time on the progress of the toning reaction.
For your conclusion describe the role of the chemical which you left out of the developer. Refer to the mechanism of the action of the black and white developer as described by your lab instructor and in the handout. Also draw a conclusion about the effect of time on the progress of the toning reaction.
1. M. Philip, Advanced Chemistry (Physical and Industrial) Published in South Asia by Foundation Books New Delhi (2003) p. 168.
2. Chris Knud-Hansen, Conflict Research Consortium (1994).