





Published on Jan 09, 2026
In the present situation energy crisis is an important unsolvable problem so we must find out some other ways to trust all sources such as solar energy, hydro power, tidal power, wind power etc. Using solar power to produce electricity is not the same as using solar to produce heat. Solar thermal principles are applied to produce hot fluids or air. Photovoltaic principles are used to produce electricity.
A solar panel (PV panel) is made of the natural element, silicon, which becomes charged electrically when subjected to sun light.
Solar panels are directed at solar south in the northern hemisphere and solar north in the southern hemisphere (these are slightly different than magnetic compass north-south directions) at an angle dictated by the geographic location and latitude of where they are to be installed. Typically, the angle of the solar array is set within a range of between site-latitude-plus 15 degrees and site-latitude-minus 15 degrees, depending on whether a slight winter or summer bias is desirable in the system. Many solar arrays are placed at an angle equal to the site latitude with no bias for seasonal periods.
This electrical charge is consolidated in the PV panel and directed to the output terminals to produce low voltage (Direct Current) - usually 6 to 24 volts. The most common output is intended for nominal 12 volts, with an effective output usually up to 17 volts. A 12 volt nominal output is the reference voltage, but the operating voltage can be 17 volts or higher much like your car alternator charges your 12 volt battery at well over 12 volts. So there's a difference between the reference voltage and the actual operating voltage.
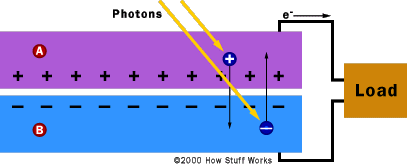
When Light Hits the Cell When light, in the form of photons, hits our solar cell, its energy frees electron-hole pairs. Each photon with enough energy will normally free exactly one electron, and result in a free hole as well. If this happens close enough to the electric field, or if free electron and free hole happen to wander into its range of influence, the field will send the electron to the N side and the hole to the P side. This causes further disruption of electrical neutrality, and if we provide an external current path, electrons will flow through the path to their original side (the P side) to unite with holes that the electric field sent there, doing work for us along the way. The electron flow provides the current, and the cell's electric field causes a voltage. With both current and voltage, we have power, which is the product of the two.

A solar cell has silicon with impurities -- other atoms mixed in with the silicon atoms, changing the way things work a bit. We usually think of impurities as something undesirable, but in our case, our cell wouldn't work without them. These impurities are actually put there on purpose. Consider silicon with an atom of phosphorous here and there, maybe one for every million silicon atoms. Phosphorous has five electrons in its outer shell, not four. It still bonds with its silicon neighbor atoms, but in a sense, the phosphorous has one electron that doesn't have anyone to hold hands with. It doesn't form part of a bond, but there is a positive proton in the phosphorous nucleus holding it in place.
A solar cell has silicon with impurities -- other atoms mixed in with the silicon atoms, changing the way things work a bit. We usually think of impurities as something undesirable, but in our case, our cell wouldn't work without them. These impurities are actually put there on purpose. Consider silicon with an atom of phosphorous here and there, maybe one for every million silicon atoms. Phosphorous has five electrons in its outer shell, not four. It still bonds with its silicon neighbor atoms, but in a sense, the phosphorous has one electron that doesn't have anyone to hold hands with. It doesn't form part of a bond, but there is a positive proton in the phosphorous nucleus holding it in place.
When energy is added to pure silicon, for example in the form of heat, it can cause a few electrons to break free of their bonds and leave their atoms. A hole is left behind in each case. These electrons then wander randomly around the crystalline lattice looking for another hole to fall into. These electrons are called free carriers, and can carry electrical current. There are so few of them in pure silicon, however, that they aren't very useful. Our impure silicon with phosphorous atoms mixed in is a different story.
It turns out that it takes a lot less energy to knock loose one of our "extra" phosphorous electrons because they aren't tied up in a bond -- their neighbors aren't holding them back. As a result, most of these electrons do break free, and we have a lot more free carriers than we would have in pure silicon. The process of adding impurities on purpose is called doping, and when doped with phosphorous, the resulting silicon is called N-type ("n" for negative) because of the prevalence of free electrons. N-type doped silicon is a much better conductor than pure silicon is.
Actually, only part of our solar cell is N-type. The other part is doped with boron, which has only three electrons in its outer shell instead of four, to become P-type silicon. Instead of having free electrons, P-type silicon ("p" for positive) has free holes. Holes really are just the absence of electrons, so they carry the opposite (positive) charge. They move around just like electrons do.
The interesting part starts when you put N-type silicon together with P-type silicon. Remember that every PV cell has at least one electric field. Without an electric field, the cell wouldn't work, and this field forms when the N-type and P-type silicon are in contact. Suddenly, the free electrons in the N side, which have been looking all over for holes to fall into, see all the free holes on the P side, and there's a mad rush to fill them in.
Before now, our silicon was all electrically neutral. Our extra electrons were balanced out by the extra protons in the phosphorous. Our missing electrons (holes) were balanced out by the missing protons in the boron. When the holes and electrons mix at the junction between N-type and P-type silicon, however, that neutrality is disrupted. Do all the free electrons fill all the free holes? No. If they did, then the whole arrangement wouldn't be very useful. Right at the junction, however, they do mix and form a barrier, making it harder and harder for electrons on the N side to cross to the P side. Eventually, equilibrium is reached, and we have an electric field separating the two sides.
We probably seen calculators that have solar cells -- calculators that never need batteries, and in some cases don't even have an off button. As long as you have enough light, they seem to work forever. You may have seen larger solar panels -- on emergency road signs or call boxes, on buoys, even in parking lots to power lights. Although these larger panels aren't as common as solar powered calculators, they're out there, and not that hard to spot if you know where to look. There are solar cell arrays on satellites, where they are used to power the electrical systems.
You have probably also been hearing about the "solar revolution" for the last 20 years -- the idea that one day we will all use free electricity from the sun. This is a seductive promise: On a bright, sunny day, the sun shines approximately 1,000 watts of energy per square meter of the planet's surface, and if we could collect all of that energy we could easily power our homes and offices for free.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |