





Published on Feb 14, 2025
The world today consumes a large amount of energy. Most of the energy requirements are fulfilled using conventional sources of energy. Of this energy consumed, a large part is utilized by the automotive sector. If the people continue using the conventional sources of energy at this rate, the earth will be facing an energy crisis very soon. The introduction of an efficient electric vehicle can greatly improve the conditions of today by helping curb the use of traditional fuels.
The Hy-Wire, discussed in this paper, runs on the electricity generated by a hydrogen fuel cell, more accurately called the 'Proton Exchange Membrane' fuel cell. This fuel cell uses hydrogen as a source of fuel. The fuel cell produces dc voltage, which is converted to ac voltage and used to run an ac motor.
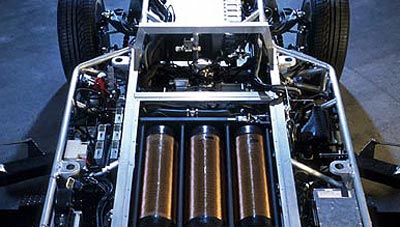
The by-wire concept removes the mechanical linkages and replaces all of them by wires and electromechanical actuators. This makes the whole vehicle lighter and more spacious. In the Hy-Wire vehicle, the whole system has been modeled into an 11-inch thick chassis. This chassis houses all the electrical components and mechanical components of the vehicle. This lets us make the body in a customized version and also lets us change the chassis architecture with radical new designs.
The by-wire system is made practical by the higher voltages inherent in a fuel cell system. The 42-V technology is made use of in this vehicle. It is said to be a luxury car in the sense that it provides the space and visibility that a luxury car does.
A fuel cell is an electrochemical energy conversion device. A fuel cell converts the hydrogen and oxygen into water and in the process produces electricity. Such fuel cells, which use hydrogen as a source of fuel, are called hydrogen fuel cells. The other electrochemical device that we are all familiar with is the battery. A battery has all of its chemicals stored inside, and it converts those chemicals into electricity too. This means that a battery eventually goes dead and you either throw it away or recharge it. With a fuel cell, chemicals constantly flow into the cell so it never goes dead - as long as there is a flow of chemicals into the cell, the electricity flows out of the cell.
Sir William Grove invented the first fuel cell in 1839. He used dilute sulphuric acid as electrolyte, oxygen as the oxidizing agent and hydrogen as fuel. In 1959, Francis T Bacon came up with an alkaline fuel cell, but it could produce only 5-kilowatt power.
A fuel cell produces dc voltage that can be used for various needs. The fuel cells are classified into various types depending upon the electrolyte they use. They are classified as follows: -
a) Direct method fuel cells
b) Solid oxide fuel cells
c) Phosphoric acid fuel cells
d) Alkaline fuel cells
e) Molten carbonate fuel cells

The "Hy" in Hy-wire stands for hydrogen, the standard fuel for a fuel cell system. Like batteries, fuel cells have a negatively charged terminal and a positively charged terminal that propel electrical charge through a circuit connected to each end. They are also similar to batteries in that they generate electricity from a chemical reaction. But unlike a battery, you can continually recharge a fuel cell by adding chemical fuel -- in this case, hydrogen from an onboard storage tank and oxygen from the atmosphere.
The basic idea is to use a catalyst to split a hydrogen molecule (H2) into two H protons (H+, positively charged single hydrogen atoms) and two electrons (e-). Oxygen on the cathode (positively charged) side of the fuel cell draws H+ ions from the anode side through a proton exchange membrane, but blocks the flow of electrons. The electrons (which have a negative charge) are attracted to the protons (which have a positive charge) on the other side of the membrane, but they have to move through the electrical circuit to get there. The moving electrons make up the electrical current that powers the various loads in the circuit, such as motors and the computer system. On the cathode side of the cell, the hydrogen, oxygen and free electrons combine to form water (H2O), the system's only emission product.
In ahydrogen fuel cell, a catalyst breaks hydrogen molecules in the anode into protons and electrons. The protons move through the exchange membrane, toward the oxygen on the cathode side, and the electrons make their way through a wire between the anode and cathode. On the cathode side, the hydrogen and oxygen combine to form water. Many cells are connected in series to move substantial charge through a circuit.
In a hydrogen fuel cell, a catalyst breaks hydrogen molecules in the anode into protons and electrons. The protons move through the exchange membrane, toward the oxygen on the cathode side, and the electrons make their way through a wire between the anode and cathode. On the cathode side, the hydrogen and oxygen combine to form water. Many cells are connected in series to move substantial charge through a circuit.
One fuel cell only puts out a little bit of power, so you need to combine many cells into a stack to get much use out of the process. The fuel-cell stack in the Hy-wire is made up of 200 individual cells connected in series, which collectively provide 94 kilowatts of continuous power and 129 kilowatts at peak power. The compact cell stack (it's about the size of a PC tower) is kept cool by a conventional radiator system that's powered by the fuel cells themselves.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |