





Published on Apr 02, 2024
Aim is to study the effect of addition of sodium carbonate on foaming capacity of a soap. Soaps and detergents are cleaning ingredients that are able to remove oil particles from surfaces because of their unique chemical properties. Soaps are created by the chemical reaction of a jetty acid with on alkali metal hydroxide. In a chemical sense soap is a salt made up of a corboxylix acid and an alkali like sodium of potassium.
The cleaning action of soap and detergents is a result of thrill, ability to surround oil particles on a surface and disperse it in water. Bar soap has been used for centuries and continues to be an important product for batching and cleaning. It is also a mild antiseptic and ingestible antidote for certain poisons. SOAP Soap is a common term for a number of related compounds used as of washing clothes or bathing. Soaps are sodium or potassium salts of higher fatty acids such as stearic acid (C17 H35 COOH), palmittic acid (C15 H31 COOH) and oleic acid (C17H35 COOH) they have the general formula RCOONa and R COONa. Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Currently sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and convert it to the salt..
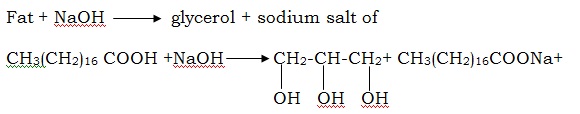
Although the reaction is shown as one step reaction, it is in fact two steps. The net effect as that the ester bonds all broken. The glycerol turns back into an alcohol. The fatty acid is turned into a salt due to the presence of abasic solution of NaoH. In the carboxyl group, one oxygen now has a negative charge that attacts the positive sodium ion. A molecule of soap consists of two parts.
a) Alkyl group – it is oil soluble
b) Corboxyl group – It is water soluble
The type of fatty acid and length of the carbon chain determines the unique properties of various soaps. Tallow or animal fats give plimarily sodium stearate (18 carbons) a very hard, insoluble soap. Fatty acids with longer chains are even more insoluble. As a matter of fact, 3inc stearate is used in talcum powders because it is water repellent. Coconut oil is a source of lauric acid (12 carbons) which can be made into sodium lourate. This soap is very soluble and will lather easily even in sea water. Fatty acids with only 10 or fewer carbons are not used in soaps because they irritate the skin and have objectionable odors
(a) Apparatus One 100ml conical flask, 20ml test tubes, 100ml measuring cylinder, test tube stand, weight box, stop watch and burner.
(b) Chemicals Soap samples, distilled water, tap water and m/10 Na2Co3 solution.
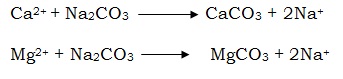
Calcium and magnesium ions present in the tap water interfere in the foaming capacity of soap. These ions combine with soap and form insoluble calcium and magnesium salts which get precipitated
![]()
Therefore, the presence of these ions effect the foaming capacity of soap and hence their cleaning capacity. When Na2CO3 is added to the tap water, calcium and magnesium ions gets precipitated as their carbonates in the presence of Na2CO3
Foaming capacity of the water increases. In order determine the effect of NO2CO3 on the foaming capacity of asample of soap it is first shaken with distrilled water there with top water and finally with top water containing equal volume of M/10 Na2CO3 solution and then the time taken for siroppealance of foam it noted
1. Weigh accurately 0.5g of the given amount of soap and transfer to a 100ml of conical flask. Add 50ml of distilled water and wolm to dissolve till clear solution is obtained.
2. Take three 20ml test tubes and label them as 1,2 and A,B and C. To test tube A add 10ml of distilled water, to test tube C add 5ml of tap water 5ml of M/10 Na2CO3 solution.
3. Add 1ml of soap solution to each tube.
4. Cork test tube A tightly and shake vigorously for 1minute. Place the test tube on the test tube stand and start the stop watch immediately. Note the taken for the disappearance of foam.
5. Repeat the same procedure for test tube B and C, rate the time taken for the disappearance of foam
Weight of soap taken = 0.5g
Volume of distilled water taken for propering solution = 50ml

• Foaming capacity of tap water increases on addition of Na2CO3 solution.